Pyruvate Carboxylase in Macrophages Aggravates Atherosclerosis by Regulating Metabolism Reprogramming to Promote Inflammatory Responses Through the Hypoxia-Inducible Factor-1 Signaling Pathway
- PMID: 40391718
- PMCID: PMC12362773
- DOI: 10.1002/advs.202417128
Pyruvate Carboxylase in Macrophages Aggravates Atherosclerosis by Regulating Metabolism Reprogramming to Promote Inflammatory Responses Through the Hypoxia-Inducible Factor-1 Signaling Pathway
Abstract
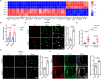
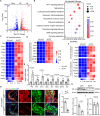
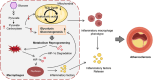
Atherosclerosis (AS) is a major cause of cardiovascular diseases, driven by chronic inflammation and macrophage polarization toward a proinflammatory phenotype. Pyruvate carboxylase (PC), a mitochondrial enzyme involved in glucose metabolism, is implicated in various metabolic disorders; however, its role in AS remains unclear. This study aims to investigate the role and mechanism of PC on macrophages in AS. PC is upregulated in macrophages of humans and mice with AS. Myeloid cell-specific PC knockout mice are generated to investigate the effects of PC deletion on atherosclerotic plaque formation. Myeloid cell-specific PC deficiency mitigates high-fat diet-induced atherosclerotic lesions in apolipoprotein E knockout mice and mice injected with adeno-associated virus-PCSK9DY. PC deletion enhances mitochondrial respiration and reduces glycolytic activity, thereby reducing reactive oxygen species overproduction and mitochondrial damage in macrophages. PC activates the hypoxia-inducible factor-1 (HIF-1) signaling pathway through macrophage metabolic reprogramming. PC induces nuclear translocation of HIF-1α in atherosclerotic aortic roots by preventing HIF-1α from proteasome degradation. HIF-1α stabilizer reverses the anti-inflammatory effect of macrophage-PC ablation in atherogenesis; however, inhibiting HIF-1α suppresses the proinflammatory macrophage phenotype induced by PC overexpression. This study indicates that macrophage PC aggravates AS through macrophage metabolic reprogramming, promoting inflammatory responses in macrophages through the HIF-1 signaling pathway.
Keywords: atherosclerosis; macrophages; metabolism reprogramming; mitochondria; pyruvate carboxylase.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
CNP Ameliorates Macrophage Inflammatory Response and Atherosclerosis.Circ Res. 2024 Apr 12;134(8):e72-e91. doi: 10.1161/CIRCRESAHA.123.324086. Epub 2024 Mar 8. Circ Res. 2024. PMID: 38456298
-
KunMingShanHaiTang formula reprograms macrophage metabolism and promotes M2 polarization via the HIF-1α pathway to alleviate ulcerative colitis symptoms in a rat model.J Bioenerg Biomembr. 2025 Jun;57(2-3):119-145. doi: 10.1007/s10863-025-10056-z. Epub 2025 Apr 2. J Bioenerg Biomembr. 2025. PMID: 40172736 Free PMC article.
-
Exosomes Derived From Human Gingival Mesenchymal Stem Cells Induce Metabolic Reprogramming of Inflammatory Macrophages.J Clin Periodontol. 2025 Aug;52(8):1196-1210. doi: 10.1111/jcpe.14184. Epub 2025 May 19. J Clin Periodontol. 2025. PMID: 40388972
-
The Bidirectional Role of Hypoxia-Inducible Factor 1 Alpha in Vascular Dementia Caused by Chronic Cerebral Hypoperfusion.Mol Neurobiol. 2025 Aug;62(8):10398-10413. doi: 10.1007/s12035-025-04914-5. Epub 2025 Apr 9. Mol Neurobiol. 2025. PMID: 40205304 Review.
-
Effect of hypoxia-inducible factor 1 on vascular endothelial growth factor expression in exercised human skeletal muscle: a systematic review and meta-analysis.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C272-C282. doi: 10.1152/ajpcell.00297.2025. Epub 2025 Jun 16. Am J Physiol Cell Physiol. 2025. PMID: 40522862 Review.
References
-
- Arnett D. K., Blumenthal R. S., Albert M. A., Buroker A. B., Goldberger Z. D., Hahn E. J., Himmelfarb C. D., Khera A., Lloyd‐Jones D., McEvoy J. W., Michos E. D., Miedema M. D., Muñoz D., Smith S. C., Virani S. S., Williams K. A., Yeboah J., Ziaeian B., J. Am. Coll. Cardiol. 2019, 74, 177. - PMC - PubMed
-
- Stary H. C., Chandler A. B., Dinsmore R. E., Fuster V., Glagov S., Insull W., Rosenfeld M. E., Schwartz C. J., Wagner W. D., Wissler R. W., Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1512. - PubMed
-
- Libby P., Ridker P. M., Maseri A., Circulation 2002, 105, 1135. - PubMed
MeSH terms
Substances
Grants and funding
- 82170274/National Natural Science Foundation of China
- 82470418/National Natural Science Foundation of China
- 82200447/National Natural Science Foundation of China
- 82470476/National Natural Science Foundation of China
- 82304477/National Natural Science Foundation of China
- 2022A0505050036/International Science and Technology Cooperation Project of Guangdong Province
- 2022A1515011747/Basic and Applied Basic Research Foundation of Guangdong Province
- 2024A1515013074/Basic and Applied Basic Research Foundation of Guangdong Province
- B2024020/Guangdong Medical Science Foundation
- 202206010065/Guangzhou Key Research and Development Program
- 2024A04J5091/Guangzhou Basic and Applied Basic Research Foundation
- 2023J004/Outstanding Youth Development Scheme of Nanfang Hospital of Southern Medical University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
