Chromosome Segregation in Closed Mitosis Under an Excess of Nuclear Envelope
- PMID: 40391723
- PMCID: PMC12090705
- DOI: 10.1111/boc.70011
Chromosome Segregation in Closed Mitosis Under an Excess of Nuclear Envelope
Abstract
Background: Two major types of cell division occur in eukaryotic cells regarding the dismantlement or not of the nuclear envelope (NE) in mitosis, open and closed mitosis, respectively. In the budding yeast Saccharomyces cerevisiae, the prototypical model for closed mitosis, the Nem1-Spo7 phosphatase complex, which regulates lipid metabolism, plays a key role in coordinating NE expansion throughout the cell cycle. Indeed, Nem1 depletion leads to abnormal NE evaginations in interphase, which protrude the ribosomal DNA (rDNA) and the nucleolus. However, the specific impact of these NE and chromosome organization abnormalities during chromosome segregation in anaphase remains poorly understood.
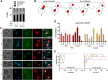
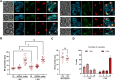
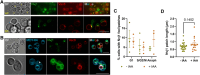
Results: Our study investigated chromosome segregation and NE dynamics during closed mitosis in relation to the presence or absence of Nem1. Nem1 was depleted by means of the auxin degron system. Nem1 depletion led to the formation of chromatin protrusions in interphase, particularly at the rDNA locus, as it has been reported before for nem1 mutants. These protrusions persisted into anaphase and were associated with delayed recoiling of the rDNA-bearing chromosome XII right arm, resulting in lagging chromatin during late anaphase. Additionally, cells can maintain nucleus-vacuole junctions (NVJs) during anaphase, suggesting that vacuoles may play a role in shaping NE morphology during chromosome segregation.
Conclusion: Our findings suggest that the Nem1-Spo7/lipin regulation of the NE size is crucial for the timely segregation of the rDNA-bearing chromosome during closed mitosis. Thus, the NE homeostasis actively contributes to chromosome segregation and the spatial organization of chromosomes in subsequent cell cycles. In addition, the persistent association between the NE and vacuoles in anaphase further underscores how cumbersome organelle interactions can become during closed mitosis, opening inspiring research avenues.
Keywords: Lipin; Nem1; Nvj1; Saccharomyces cerevisiae; chromosome segregation; closed mitosis; nuclear envelope; rDNA; vacuole.
© 2025 The Author(s). Biology of the Cell published by Wiley‐VCH GmbH on behalf of Société Française des Microscopies and Société de Biologie Cellulaire de France.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The yeast polo kinase Cdc5 regulates the shape of the mitotic nucleus.Curr Biol. 2014 Dec 1;24(23):2861-7. doi: 10.1016/j.cub.2014.10.029. Epub 2014 Nov 20. Curr Biol. 2014. PMID: 25454593 Free PMC article.
-
The mode of mitosis is dramatically modified by deletion of a single nuclear pore complex gene in Aspergillus nidulans.Fungal Genet Biol. 2019 Sep;130:72-81. doi: 10.1016/j.fgb.2019.04.010. Epub 2019 Apr 23. Fungal Genet Biol. 2019. PMID: 31026588
-
SUMOylation at the inner nuclear membrane facilitates nuclear envelope biogenesis during mitosis.J Cell Biol. 2023 Aug 7;222(8):e202208137. doi: 10.1083/jcb.202208137. Epub 2023 Jul 3. J Cell Biol. 2023. PMID: 37398994 Free PMC article.
-
Cdc14 and the temporal coordination between mitotic exit and chromosome segregation.Cell Cycle. 2005 Jan;4(1):109-12. doi: 10.4161/cc.4.1.1356. Epub 2005 Jan 10. Cell Cycle. 2005. PMID: 15611663 Review.
-
A case of selfish nucleolar segregation.Cell Cycle. 2005 Jan;4(1):113-7. doi: 10.4161/cc.4.1.1488. Epub 2005 Jan 21. Cell Cycle. 2005. PMID: 15655374 Review.
References
-
- Amberg, D. C. , Burke D., and Strathern J.. 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. 2005 ed., c2005. Cold Spring Harbor Laboratory Press.
MeSH terms
Substances
Grants and funding
- PID2021-123716OB-I100/Ministerio de Ciencia, Innovación y Universidades
- FPI2024010226/Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI)
- TESIS2018010034/Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI)
- TESIS2020010028/Agencia Canaria de Investigación, Innovación y Sociedad de la Información (ACIISI)
- PRDTF234034MORA/Asociación Española contra el Cáncer (AECC)
LinkOut - more resources
Full Text Sources

