Dual Benefits of Oral Tranexamic Acid: Reducing Melasma Severity and Inflammation
- PMID: 40391727
- PMCID: PMC12090704
- DOI: 10.1111/jocd.70257
Dual Benefits of Oral Tranexamic Acid: Reducing Melasma Severity and Inflammation
Abstract
Background: Melasma is a chronic hyperpigmentation disorder influenced by hormonal factors, ultraviolet exposure, and inflammation. While oral tranexamic acid (TXA) is an established treatment, its effects on systemic inflammation remain unclear.
Aims: This study aimed to evaluate the impact of TXA on melasma severity and inflammatory markers.
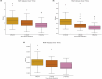
Methods: This retrospective study included 80 melasma patients and 80 healthy controls. Patients received oral TXA (500 mg/day) for 3 months. Melasma severity was assessed using the Melasma Area and Severity Index (MASI), and inflammatory markers (monocyte, neutrophil, lymphocyte, HDL, MHR, MLR, NLR) were measured at baseline, Month 1, and Month 3. Changes within the melasma group and comparisons with controls were analyzed.
Results: At 3 months, melasma severity significantly improved, with a 65.1% reduction in MASI (from 12.9 to 4.5, p < 0.001). Monocyte, neutrophil, MHR, MLR, and NLR levels significantly decreased, while HDL and lymphocyte levels increased (p < 0.001). Compared to controls, baseline inflammatory marker levels differed significantly; however, at month 3, only monocyte, MHR, and HDL remained significantly different (p < 0.05). Regression analysis identified NLR and HDL as significant predictors of melasma severity reduction (p = 0.045 and p = 0.011, respectively).
Conclusion: Oral TXA not only improved melasma severity but also modulated systemic inflammation. The association between NLR, HDL, and treatment response suggests their potential as biomarkers for monitoring therapeutic efficacy.
Keywords: high‐density lipoprotein (HDL); inflammatory markers; lymphocytes; melasma; monocytes; neutrophils; skin pigmentation disorders; tranexamic acid.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Mahajan V. K., Patil A., Blicharz L., et al., “Medical Therapies for Melasma,” Journal of Cosmetic Dermatology 21 (2022): 3707–3728. - PubMed
-
- Rajanala S., Maymone M. B. C., and Vashi N. A., “Melasma Pathogenesis: A Review of the Latest Research, Pathological Findings, and Investigational Therapies,” Dermatology Online Journal 25 (2019): 1–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

