Distinct iron acquisition strategies in oceanic and coastal variants of the mixotrophic dinoflagellate Karlodinium
- PMID: 40391772
- PMCID: PMC12161497
- DOI: 10.1093/ismejo/wraf099
Distinct iron acquisition strategies in oceanic and coastal variants of the mixotrophic dinoflagellate Karlodinium
Abstract
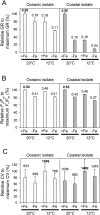
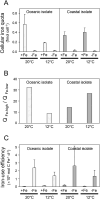
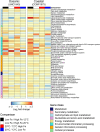
The availability of the micronutrient iron is important in regulating phytoplankton growth across much of the world's oceans, particularly in the high-nutrient, low-chlorophyll regions. Compared to known mechanisms of iron acquisition and conservation in autotrophic protists (e.g. diatoms), those of dinoflagellates remain unclear, despite their frequent presence in offshore iron-limited waters. Here, we investigate the strategies of an ecologically important mixotrophic dinoflagellate to coping with low iron conditions. Coupled gene expression and physiological responses as a function of iron availability were examined in oceanic and coastal strains of the dinoflagellate Karlodinium. Under iron-replete conditions, grazing was only detected in coastal variants, resulting in faster growth rates compared to when grown autotrophically. Under iron-limited conditions, all isolates exhibited slower growth rates, reduced photosynthetic efficiencies, and lower cellular iron quotas than in iron-replete conditions. However, oceanic isolates exhibited higher relative growth rates compared to coastal isolates under similar low iron concentrations, suggesting they are better adapted to coping under iron limitation. Yet the oceanic isolates did not exhibit the ability to appreciably reduce cell volume or increase iron-use efficiencies compared to the coastal isolates to cope with iron limitation, as often observed in oceanic diatoms. Rather, molecular pathway analysis and corresponding gene expression patterns suggest that oceanic Karlodinium utilizes a high-affinity iron uptake system when iron is low. Our findings reveal cellular mechanisms by which dinoflagellates have adapted to low iron conditions, further shedding light on how they potentially survive in variable iron regions of the world's oceans.
Keywords: dinoflagellates; iron acquisition; iron-limited regions; mixotrophy; oceanic and coastal variants; transcriptomics.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures

Similar articles
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Holocene and contemporary marine dinoflagellate community patterns predict expansion of generalist dinoflagellate blooms in warming oceans.ISME J. 2025 Jan 2;19(1):wraf095. doi: 10.1093/ismejo/wraf095. ISME J. 2025. PMID: 40367347 Free PMC article.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
References
-
- Jeong HJ, Yoo YD, Kim JS. et al. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J 2010;45:65–91. 10.1007/s12601-010-0007-2 - DOI
-
- Stoecker DK. Mixotrophy among dinoflagellates. J Eukaryot Microbiol 1999;46:397–401. 10.1111/j.1550-7408.1999.tb04619.x - DOI
-
- Gómez F. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Syst Biodivers 2012;10:267–75. 10.1080/14772000.2012.721021 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

