The Human Mechanosensory Corpuscles: A New Schwann Cell Localization of the Wilms' Tumor Protein WT1
- PMID: 40391844
- PMCID: PMC12092412
- DOI: 10.1369/00221554251338066
The Human Mechanosensory Corpuscles: A New Schwann Cell Localization of the Wilms' Tumor Protein WT1
Abstract
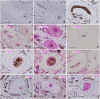
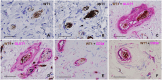
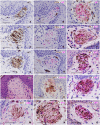
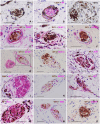
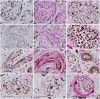
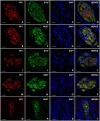
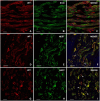
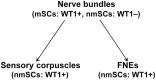
SummaryThe Wilms' Tumor protein WT1 is a zinc-finger transcription factor with crucial roles in organogenesis, cell differentiation, tissue homeostasis, and oncogenesis. While its expression has been extensively studied in various tissues, its presence in the nervous system, particularly in peripheral glial cells, remains largely unexplored. In this study, we examined WT1 expression in the Schwann cells of mechanosensory corpuscles, nerve bundles, and free nerve endings (FNEs) within human penile tissues. Using single and double immunohistology, we analyzed WT1 coexpression with Schwann cell markers (S100, nestin, SOX10) and its association with axonal (neurofilaments, neuron-specific enolase, tyrosine hydroxylase) and perineurial/endoneurial markers (Glut-1, α-SMA, CD34). We found consistent WT1 cytoplasmic expression in the Schwann cells of Pacinian, Meissner, Krause, genital, Golgi-Mazzoni, and Ruffini-like corpuscles, with variable staining intensity. Confocal microscopy revealed WT1 colocalized with nestin but not S100, suggesting involvement in cytoskeletal organization. In addition, we documented WT1 in myelinating Schwann cells of nerve bundles, with distinct staining patterns in Cajal bands and Schmidt-Lanterman incisures, as well as in non-myelinating Schwann cells of FNEs. This is the first study to describe WT1 expression in sensory corpuscles, implicating it in Schwann cell development, maintenance, or plasticity, with potential relevance for peripheral nerve biology, pathology, and mechanosensation.
Keywords: cytoskeleton; foreskin; genitalia; immunohistochemistry; mechanoreceptors; nerve endings; neuroglia; penis; peripheral nervous system; urogenital system.
Conflict of interest statement
Competing InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Cutaneous Innervation in the Red-Eared Slider Turtle: Novel Identification and Analysis of Scale Sensilla and Sensory Corpuscles With Immunofluorescence Insights and Ecological Implications.J Exp Zool A Ecol Integr Physiol. 2025 Jul 16. doi: 10.1002/jez.70014. Online ahead of print. J Exp Zool A Ecol Integr Physiol. 2025. PMID: 40667616
-
Identification of cellular and noncellular components of mature intact human peripheral nerve.J Peripher Nerv Syst. 2024 Sep;29(3):294-314. doi: 10.1111/jns.12643. Epub 2024 Jul 7. J Peripher Nerv Syst. 2024. PMID: 38973168
-
Etv5 Is Required for Peripheral Nerve Function and the Injury Response.eNeuro. 2025 Jul 23;12(7):ENEURO.0410-20.2025. doi: 10.1523/ENEURO.0410-20.2025. Print 2025 Jul. eNeuro. 2025. PMID: 40701803 Free PMC article.
-
Active specific immunotherapy targeting the Wilms' tumor protein 1 (WT1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials.Oncologist. 2012;17(2):250-9. doi: 10.1634/theoncologist.2011-0240. Epub 2012 Jan 30. Oncologist. 2012. PMID: 22291091 Free PMC article.
-
Fascial Innervation: A Systematic Review of the Literature.Int J Mol Sci. 2022 May 18;23(10):5674. doi: 10.3390/ijms23105674. Int J Mol Sci. 2022. PMID: 35628484 Free PMC article.
References
-
- Zelená J. Nerves and mechanoreceptors: the role of innervation in the development and maintenance of mammalian mechanoreceptors. London: Chapman & Hall; 1994.
-
- Malinovský L, Pác L. Morphology of sensory corpuscles in mammals. Brno: Acta Facultatis Medicae Universitatis Brunensis; 1982.
-
- Krause W. Die nervenendigungen innerhalb der terminalen korperchen [The nerve endings within the terminal corpuscles]. Arch Mikr Anat Entwgsch. 1881;19:53–136.
-
- Chouchkov C. Cutaneous receptors. New York: Springer-Verlag; 1978.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

