Microbial vitamin biosynthesis links gut microbiota dynamics to chemotherapy toxicity
- PMID: 40391895
- PMCID: PMC12153289
- DOI: 10.1128/mbio.00930-25
Microbial vitamin biosynthesis links gut microbiota dynamics to chemotherapy toxicity
Abstract
Dose-limiting toxicities pose a major barrier to cancer treatment. While preclinical studies show that the gut microbiota influences and is influenced by anticancer drugs, data from patients paired with careful side effect monitoring remains limited. Here, we investigate capecitabine (CAP)-microbiome interactions through longitudinal metagenomic sequencing of stool from 56 advanced colorectal cancer patients. CAP significantly altered the gut microbiome, enriching for menaquinol (vitamin K2) biosynthesis genes. Transposon library screens, targeted gene deletions, and media supplementation revealed that menaquinol biosynthesis protects Escherichia coli from drug toxicity. Stool menaquinol gene and metabolite levels were associated with decreased peripheral sensory neuropathy. Machine learning models trained in this cohort predicted toxicities in an independent cohort. Taken together, these results suggest treatment-associated increases in microbial vitamin biosynthesis serve a chemoprotective role for bacterial and host cells. Further, our findings provide a foundation for in-depth mechanistic dissection, human intervention studies, and extension to other cancer treatments.IMPORTANCESide effects are common during the treatment of cancer. The trillions of microbes found within the human gut are sensitive to anticancer drugs, but the effects of treatment-induced shifts in gut microbes for side effects remain poorly understood. We profiled gut microbes in colorectal cancer patients treated with capecitabine and carefully monitored side effects. We observed a marked expansion in genes for producing vitamin K2 (menaquinone). Vitamin K2 rescued gut bacterial growth and was associated with decreased side effects in patients. We then used information about gut microbes to develop a predictive model of drug toxicity that was validated in an independent cohort. These results suggest that treatment-associated increases in bacterial vitamin production protect both bacteria and host cells from drug toxicity, providing new opportunities for intervention and motivating the need to better understand how dietary intake and bacterial production of micronutrients like vitamin K2 influence cancer treatment outcomes.
Keywords: chemotherapy; colorectal cancer; human gut microbiome; metagenomics; vitamin K.
Conflict of interest statement
For conflicts of interest, see Acknowledgments.
Figures

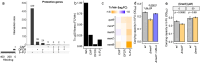


References
-
- Schuurhuizen CS, Verheul HM, Braamse AM, Buffart LM, Bloemendal HJ, Dekker J, Konings IR. 2018. The predictive value of cumulative toxicity for quality of life in patients with metastatic colorectal cancer during first-line palliative chemotherapy. Cancer Manag Res 10:3015–3021. doi: 10.2147/CMAR.S166468 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
