Population pharmacokinetics of penicillin G: insights into increased clearance at low concentrations to guide development of improved long-acting formulations for syphilis and prevention of rheumatic fever
- PMID: 40391929
- PMCID: PMC12217478
- DOI: 10.1128/aac.00269-25
Population pharmacokinetics of penicillin G: insights into increased clearance at low concentrations to guide development of improved long-acting formulations for syphilis and prevention of rheumatic fever
Abstract
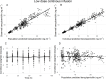
Although benzylpenicillin (penicillin G) is listed by the World Health Organization as an Essential Medicine, dose optimization is a persistent challenge, especially for long-acting intramuscular formulations. Maintaining sustained antibiotic exposure at target concentrations is crucial for secondary chemoprophylaxis of rheumatic heart disease and treatment of syphilis. This study compared the pharmacokinetic profile of continuous low-dose benzylpenicillin infusions with a standard-dose bolus and evaluated which renal function marker (serum creatinine, cystatin C, or combined e-glomerular filtration rate [eGFR]) best predicted clearance. Healthy adult volunteers received a single 600 mg IV benzylpenicillin bolus followed by randomization to continuous infusions targeting steady-state concentrations of 3, 6, 9, 12, or 20 ng/mL. Plasma benzylpenicillin concentrations were measured by liquid chromatography-mass spectrometry. Population pharmacokinetic analysis was performed using NONMEM by incorporating both bolus and infusion data, and various GFR estimations were evaluated as covariates for clearance. Data from 72 participants were analyzed, including 504 bolus and 389 continuous infusion samples. A two-compartment model improved fit when the ratio of central volume of distribution between bolus and low-dose infusion was incorporated, and clearance differences at steady state plasma concentration of 3 ng/mL were accounted for. Of the GFR estimations, cystatin C-based eGFR significantly enhanced model fit compared with creatinine-based equations. Benzylpenicillin pharmacokinetics at very low concentrations demonstrated both a higher volume of distribution and increased clearance. Cystatin C-based eGFR may more accurately predict benzylpenicillin clearance, enabling precision dosing for long-acting preparations used for treatment of syphilis and prevention of rheumatic fever.
Keywords: benzathine penicillin G; benzylpenicillin; cystatin C; eGFR; penicillin G; pharmacokinetics; population PK modeling; renal clearance; rheumatic fever; rheumatic heart disease; syphilis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- 2025. Australian medicines handbook (online). Australian medicines handbook Pty Ltd, Adelaide.
-
- ARF RHD guidelines available online. Available from: https://www.rhdaustralia.org.au/arf-rhd-guidelines. Accessed 28 December 2024
-
- Watkins D, Baker MG, Kumar RK, Parks T. 2021. Chapter 1 - Epidemiology, risk factors, burden and cost of acute rheumatic fever and rheumatic heart disease, p 1–18. In Dougherty S, Carapetis J, Zühlke L, Wilson N (ed), In acute rheumatic fever and rheumatic heart disease. Elsevier, San Diego (CA).
-
- Sexually transmitted infections (STIs). Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-in.... Accessed 22 January 2025
-
- Hla TK, Osowicki J, Marsh JA, Salman S, Page-Sharp M, Yoo O, Azzopardi K, Morici M, Batty KT, Barr RK, Enkel SL, Kado J, Hatchuel L, Fulurija A, McCarthy JS, Snelling TL, Steer AC, Carapetis J, Manning L. 2025. Establishing the lowest penicillin concentration to prevent pharyngitis due to Streptococcus pyogenes using A human challenge model (CHIPS): A randomised, double-blind, placebo-controlled trial. The Lancet Microbe 101038:101038. doi: 10.1016/j.lanmic.2024.101038 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

