Metformin as antiviral therapy protects hyperglycemic and diabetic patients
- PMID: 40391966
- PMCID: PMC12153287
- DOI: 10.1128/mbio.00634-25
Metformin as antiviral therapy protects hyperglycemic and diabetic patients
Abstract
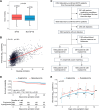
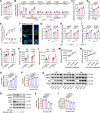
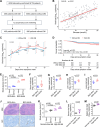
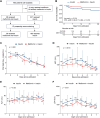
Viral infections disrupt glucose metabolism; however, their impact on disease prognosis in highly pathogenic viruses remains largely unknown. There is an additional need to investigate the antiviral mechanisms of glucose-lowering therapeutics. Here, our multicenter clinical study shows that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients infected with severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging and highly pathogenic bunyavirus. SFTSV infection triggers gluconeogenesis, which, in turn, inhibits AMPK activity and subsequent interferon I (IFN-I) responses, thereby facilitating viral replication. In vitro and animal studies further reveal that metformin inhibits SFTSV replication by suppressing autophagy through the AMPK-mTOR pathway, contributing to protection against lethal SFTSV infection in mice. Importantly, our large cohort study demonstrates that metformin reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, contrasting with the disadvantageous effect of insulin. These findings highlight the promising therapeutic potential of metformin in treating viral infections, particularly among individuals with hyperglycemia or diabetes.
Importance: Severe fever with thrombocytopenia syndrome virus (SFTSV), an emerging tick-borne bunyavirus, causes severe hemorrhagic fever with a high mortality rate. Previous studies have shown metabolic disturbances, particularly hyperglycemia, in SFTSV-infected individuals. However, the mechanism underlying this metabolic derangement remains unclear, and further investigation is needed to determine whether glucose-lowering therapeutics could be beneficial for SFTSV-infected patients. In this study, our multicenter clinical data show that hyperglycemia and pre-existing diabetes are independent risk factors for mortality in patients with SFTSV infection. Furthermore, we observed that SFTSV infection triggers gluconeogenesis, which promotes viral replication through the regulation of the AMPK-IFN-I signaling pathway. Notably, metformin significantly reduces viremia and SFTSV-related mortality in patients with hyperglycemia or pre-existing diabetes, attributed to its inhibitory effect on autophagy through the AMPK-mTOR pathway. Therefore, our study uncovers the interaction between SFTSV infection and glucose metabolic disorder and highlights the promising therapeutic potential of metformin for treating SFTSV infection.
Keywords: antiviral therapy; diabetes; hyperglycemia; metformin; severe fever with thrombocytopenia syndrome virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oral anti-diabetic agents for women with established diabetes/impaired glucose tolerance or previous gestational diabetes planning pregnancy, or pregnant women with pre-existing diabetes.Cochrane Database Syst Rev. 2017 Oct 18;10(10):CD007724. doi: 10.1002/14651858.CD007724.pub3. Cochrane Database Syst Rev. 2017. PMID: 29045765 Free PMC article.
-
Endothelial cell-released mitochondrial DNA promotes B cell differentiation and virus replication during severe fever with thrombocytopenia syndrome virus infection.J Virol. 2025 Jun 17;99(6):e0132324. doi: 10.1128/jvi.01323-24. Epub 2025 May 14. J Virol. 2025. PMID: 40366175 Free PMC article.
-
Identification of Licoflavone C as a cap-dependent endonuclease inhibitor against severe fever with thrombocytopenia syndrome virus.Acta Pharmacol Sin. 2025 Sep;46(9):2482-2495. doi: 10.1038/s41401-025-01533-7. Epub 2025 Apr 1. Acta Pharmacol Sin. 2025. PMID: 40169781
-
Bergamottin, A Natural Bioactive Compound, Inhibits Dabie Bandavirus Infection In Vitro and In Vivo.J Med Virol. 2025 Jul;97(7):e70495. doi: 10.1002/jmv.70495. J Med Virol. 2025. PMID: 40673712
-
Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility.Cochrane Database Syst Rev. 2017 Nov 29;11(11):CD003053. doi: 10.1002/14651858.CD003053.pub6. Cochrane Database Syst Rev. 2017. PMID: 29183107 Free PMC article.
References
-
- Singh S, Singh PK, Suhail H, Arumugaswami V, Pellett PE, Giri S, Kumar A. 2020. AMP-activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis. J Immunol 204:1810–1824. doi: 10.4049/jimmunol.1901310 - DOI - PMC - PubMed
-
- Barreto EA, Cruz AS, Veras FP, Martins R, Bernardelli RS, Paiva IM, Lima TM, Singh Y, Guimarães RC, Damasceno S, et al. 2023. COVID-19-related hyperglycemia is associated with infection of hepatocytes and stimulation of gluconeogenesis. Proc Natl Acad Sci USA 120:e2217119120. doi: 10.1073/pnas.2217119120 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

