Microbiome and metabolomic changes associated with HPV clearance in women undergoing local excisional treatment for cervical intraepithelial neoplasia
- PMID: 40391990
- PMCID: PMC12172484
- DOI: 10.1128/msystems.00511-25
Microbiome and metabolomic changes associated with HPV clearance in women undergoing local excisional treatment for cervical intraepithelial neoplasia
Abstract
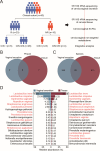
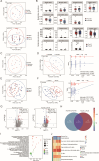
Cervical intraepithelial neoplasia (CIN) is a common gynecological condition often associated with persistent human papillomavirus (HPV) infections. Although the loop electrosurgical excision procedure (LEEP) is effective in removing lesions, some patients remain HPV positive post-treatment. In this prospective study, we enrolled reproductive-age women diagnosed with HPV-related CIN and employed a multi-omics analysis of cervicovaginal secretion and cervical tissue microbiomes, alongside non-targeted and targeted metabolomic assessments. We aim to explore the role of the cervicovaginal and intratissue microbiota and associated metabolites on HPV clearance following LEEP. We observed significant shifts in bacterial diversity and composition in both cervicovaginal secretion and cervical tissue samples. Notably, distinct bacterial species, such as Lactobacillus and certain anaerobes (e.g., Prevotella bivia), were correlated with HPV clearance post-LEEP. Metabolomic profiling revealed that the HPV-cleared group exhibited elevated acetic acid levels and significant alterations in glycerophospholipid metabolism, suggesting a potential role in promoting HPV clearance. Correlation analyses demonstrated significant associations between altered bacteria and metabolites with HPV status, with models incorporating both achieving high predictive accuracy. Overall, our findings highlight the importance of the cervicovaginal and intratissue microbiomes and metabolites in facilitating HPV clearance, suggesting potential therapeutic targets for patients with CIN.
Importance: The clearance of human papillomavirus (HPV) after local excisional treatment for cervical intraepithelial neoplasia is crucial for patient health. This study reveals significant alterations in the cervicovaginal secretion and cervical tissue microbiomes, alongside metabolomic changes, which are associated with HPV clearance. Through a comprehensive multi-omics approach, we identified specific bacterial species and metabolic changes that correlate with successful HPV clearance post-loop electrosurgical excision procedure. Notably, the presence of beneficial Lactobacillus species and elevated levels of acetic acid linked to glycerophospholipid metabolism emerged as potential biomarkers for HPV status, suggesting that these factors play a pivotal role in improving treatment outcomes. These findings highlight the potential for microbiome-targeted therapies to enhance HPV clearance and provide insights into the microbial and metabolic mechanisms involved in cervical health.
Keywords: HPV; cervical intraepithelial neoplasia; metabolome; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Xia W, Dai X, Hu Y, Yang S, Chen C, Li X. 2024. Value of intraoperative post-conisation human papillomavirus testing in predicting residual or recurrence after treatment with a loop electrosurgical excision procedure in women with HR-HPV positive and cervical high-grade squamous intraepithelial lesion. BMC Cancer 24:1496. doi: 10.1186/s12885-024-13272-9 - DOI - PMC - PubMed
-
- Wiik J, Sengpiel V, Kyrgiou M, Nilsson S, Mitra A, Tanbo T, Monceyron Jonassen C, Møller Tannæs T, Sjøborg K. 2019. Cervical microbiota in women with cervical intra-epithelial neoplasia, prior to and after local excisional treatment, a Norwegian cohort study. BMC Womens Health 19:30. doi: 10.1186/s12905-019-0727-0 - DOI - PMC - PubMed
-
- Kawahara R, Fujii T, Kukimoto I, Nomura H, Kawasaki R, Nishio E, Ichikawa R, Tsukamoto T, Iwata A. 2021. Changes to the cervicovaginal microbiota and cervical cytokine profile following surgery for cervical intraepithelial neoplasia. Sci Rep 11:2156. doi: 10.1038/s41598-020-80176-6 - DOI - PMC - PubMed
-
- Bénard EA, Carceller AM, Mayrand M-H, Lacroix J, Niyibizi J, Laporte L, Comète E, Coutlée F, Trottier H, HERITAGE study group . 2025. Viral Load of Human Papillomavirus (HPV) during pregnancy and its association with HPV vertical transmission. J Med Virol 97:e70221. doi: 10.1002/jmv.70221 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

