Hybrid B- and T-Cell Immunity Associates With Protection Against Breakthrough Infection After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Avon Longitudinal Study of Parents and Children (ALSPAC) Participants
- PMID: 40392230
- PMCID: PMC12349941
- DOI: 10.1093/infdis/jiaf246
Hybrid B- and T-Cell Immunity Associates With Protection Against Breakthrough Infection After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Avon Longitudinal Study of Parents and Children (ALSPAC) Participants
Abstract
Background: Immunological memory to vaccination and viral infection involves the coordinated action of B and T cells; thus, integrated analysis of these 2 components is critical for understanding their respective contributions to protection against breakthrough infections (BIs) after vaccination.
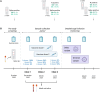
Methods: We investigated cellular and humoral immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and/or vaccination in 300 adult participants from the Avon Longitudinal Study of Parents and Children (ALSPAC). Participants were grouped by those with (cases) and without (controls) a history of SARS-CoV-2 infection. To provide a quantitative correlate for protection against BI in the 8-month period after the study, Youden index thresholds were calculated for all immune measures analyzed.
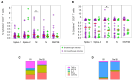
Results: The magnitude of antibody and T-cell responses following the second vaccine dose was associated with protection against BI in participants with a history of SARS-CoV-2 infection (cases), but not in infection-naive controls. Over 8 months of follow-up, 2 threshold combinations provided the best performance for protection against BI in cases: (i) anti-spike immunoglobulin G (IgG) (≥666.4 binding antibody units [BAU]/mL) combined with anti-nucleocapsid pan-immunoglobulin (pan-Ig) (≥0.1332 BAU/mL) and (ii) spike 1-specific T cells (≥195.6 spot-forming units/106 peripheral blood mononuclear cells) combined with anti-N pan-Ig (≥0.1332 BAU/mL). Both combinations offered 100% specificity for detecting cases without BI, with sensitivities of 83.3% and 72.2%, respectively.
Conclusions: Collectively, these results suggest that hybrid B- and T-cell immunity offers superior protection from BI after coronavirus disease 2019 (COVID-19) vaccination, and this finding has implications for designing next-generation COVID-19 vaccines that are capable of eliciting immunity to a broader repertoire of SARS-CoV-2 proteins.
Keywords: ALSPAC; SARS-CoV-2; breakthrough infection; hybrid immunity; vaccination.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. F. was a lead investigator on trials of COVID-19 vaccines funded by Oxford/AstraZeneca, Valneva, Sanofi, and the UK government. He also leads a University of Bristol–sponsored epidemiological study of adult respiratory disease funded by Pfizer, which has evaluated COVID-19 vaccine effectiveness. During the pandemic he was a member of the Joint Committee on Vaccination and Immunisation, which advised the UK government on COVID-19 vaccine policy, and of the World Health Organization Specialist Advisory Group of Experts COVID-19 vaccine working group. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures




References
-
- Desantis SM, Yaseen A, Hao T, et al. Incidence and predictors of breakthrough and severe breakthrough infections of SARS-CoV-2 after primary series vaccination in adults: a population-based survey of 22 575 participants. J Infect Dis 2023; 227:1164–72. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 217065/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- SB007/100173/AMS_/Academy of Medical Sciences/United Kingdom
- C18281/A29019/CRUK Integrative Cancer Epidemiology Programme
- University's Alumni and Friends
- 102215/2/13/2/WT_/Wellcome Trust/United Kingdom
- ALSPAC
- WT_/Wellcome Trust/United Kingdom
- WT 217065/Z/19/Z/MRC_/Medical Research Council/United Kingdom
- University of Bristol
- MC_UU_00011/1/Integrative Epidemiology Unit
- MR/V028448/1/UK Research and Innovation
- BRC-1215-2001/University of Bristol National Institute for Health and Care Research Biomedical Research Centre
- Elizabeth Blackwell Institute
- MR/W021315/1/MRC
- 204813/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- Avon Longitudinal Study of Parents and Children (ALSPAC)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

