A retrospective study of kidney disease in Alport syndrome during and after pregnancy
- PMID: 40392259
- PMCID: PMC12165875
- DOI: 10.1007/s40620-025-02252-2
A retrospective study of kidney disease in Alport syndrome during and after pregnancy
Abstract
Background: During pregnancy, hyperfiltration and other factors are hypothesized to contribute to the progression of kidney disease in women with Alport syndrome. To evaluate the status of kidney disease, clinical data from mothers with Alport syndrome in China and Europe over the pregnancy were analyzed.
Methods: This retrospective observational study collected data to evaluate proteinuria, kidney function and Alport stage prior to, during, and after pregnancy, respectively.
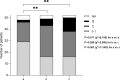
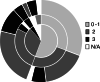
Results: A total of 74 women were enrolled, 82% of them with X-linked Alport syndrome and 11% with autosomal Alport syndrome (unknown in 5 patients). Detailed information on the course of pregnancy was available for 62 pregnancies from 52 different women. No fetal malformations were observed. Mean gestational age was 37.9 ± 2.7 weeks (n = 55). Complications included high blood pressure (n = 8), abortion (n = 5), preeclampsia (n = 5), gestational diabetes (n = 3), nephrotic syndrome (n = 2), cervical insufficiency with fetal growth delay (n = 2), premature rupture of membranes (n = 1) and acute intrauterine fetal distress (n = 1). Median proteinuria was 350 (30-2465) mg/day prior to pregnancy, 2390 (450-11,450) mg/day during pregnancy, and 590 (40-2650) mg/day at a mean postpartum follow-up time of 4.5 ± 2.1 years. Mean estimated glomerular filtration rate (eGFR) decreased by 17.2 ± 16.7 ml/min/1.73 m2, from 96.1 ± 32.9 to 78.9 ± 37 ml/min/1.73 m2 after pregnancy (n = 15; p = 0.003). The eGFR loss was higher in women with eGFR < 90 ml/min/1.73 m2 prior to pregnancy compared to women with normal renal function (- 21.5 ± 9.8 vs. - 14 ± 20 ml/min/1.73 m2), and in women with severe variants compared to women with less severe variants (- 21.5 ± 20.2 vs. - 11.3 ± 19.0 ml/min/1.73 m2). Progression of Alport stage after pregnancy was observed in 42% of the women, 31% remained in stage 0-1, and 23% remained in stage 2.
Conclusions: This study provides important data on the natural history of Alport syndrome in women who have undergone a pregnancy. Women with severe variants of Alport syndrome, and women with eGFR below 90 ml/min/1.73 m2 face greater risks of kidney disease progression after pregnancy. Further prospective studies are required to confirm these findings.
Keywords: Alport syndrome; Pregnancy; Proteinuria; Women.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: No conflicts of interest are declared by the authors. Ethical approval: This observational, retrospective study was conducted in accordance with the declaration of Helsinki. Data acquisition in China was reviewed and approved by the Institutional Review Board of Peking University First Hospital (2022 [051] V1.0). Informed consent for this study was obtained from all patients. Data acquisition in Europe was part of the European Alport registry, which has been reviewed and approved by the Institutional Review Board of University Medicine Goettingen (AZ 10/11/06). Statement of human and animal rights: All procedures were approved by the Peking University First Hospital Institutional Review Board (2022 [051] V1.0) and the University Medicine Goettingen Institutional Review Board (AZ 10/11/06). Informed consent: Informed consent was obtained from all individual participants.
Figures


Similar articles
-
Pregnancy outcomes in patients with Alport syndrome.Arch Gynecol Obstet. 2016 Apr;293(4):739-47. doi: 10.1007/s00404-015-3893-9. Epub 2015 Sep 28. Arch Gynecol Obstet. 2016. PMID: 26411580
-
Reassuring pregnancy outcomes in women with mild COL4A3-5-related disease (Alport syndrome) and genetic type of disease can aid personalized counseling.Kidney Int. 2024 May;105(5):1088-1099. doi: 10.1016/j.kint.2024.01.034. Epub 2024 Feb 19. Kidney Int. 2024. PMID: 38382843
-
Losartan and enalapril are comparable in reducing proteinuria in children with Alport syndrome.Pediatr Nephrol. 2013 May;28(5):737-43. doi: 10.1007/s00467-012-2372-9. Epub 2012 Dec 4. Pediatr Nephrol. 2013. PMID: 23207876 Clinical Trial.
-
Pregnancy in women with Alport syndrome.Int Urol Nephrol. 2013 Aug;45(4):1223-7. doi: 10.1007/s11255-012-0154-8. Epub 2012 Mar 15. Int Urol Nephrol. 2013. PMID: 22418765 Review.
-
Alport syndrome and pregnancy: a case series and literature review.Arch Gynecol Obstet. 2018 Jun;297(6):1421-1431. doi: 10.1007/s00404-018-4720-x. Epub 2018 Feb 28. Arch Gynecol Obstet. 2018. PMID: 29492669 Review.
References
-
- Imbasciati E, Gregorini G, Cabiddu G et al (2007) Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis 49(6):753–762 - PubMed
-
- Kashtan CE, Ding J, Garosi G et al (2018) Alport syndrome: a unified classification of genetic disorders of collagen IV alpha345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int 93(5):1045–1051 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

