Caspase Domain Duplication During the Evolution of Caspase-16
- PMID: 40392285
- PMCID: PMC12198278
- DOI: 10.1007/s00239-025-10252-w
Caspase Domain Duplication During the Evolution of Caspase-16
Abstract
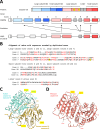
Caspases are cysteine-dependent aspartate-directed proteases which have critical functions in programmed cell death and inflammation. Their catalytic activity depends on a catalytic dyad of cysteine and histidine within a characteristic protein fold, the so-called caspase domain. Here, we investigated the evolution of caspase-16 (CASP16), an enigmatic member of the caspase family, for which only a partial human gene had been reported previously. The presence of CASP16 orthologs in placental mammals, marsupials and monotremes suggests that caspase-16 originated prior to the divergence of the main phylogenetic clades of mammals. Caspase-16 proteins of various species contain a carboxy-terminal caspase domain and an amino-terminal prodomain predicted to fold into a caspase domain-like structure, which is a unique feature among caspases known so far. Comparative sequence analysis indicates that the prodomain of caspase-16 has evolved by the duplication of exons encoding the caspase domain, whereby the catalytic site was lost in the amino-terminal domain and conserved in the carboxy-terminal domain of caspase-16. The murine and human orthologs of CASP16 contain frameshift mutations and therefore represent pseudogenes (CASP16P). CASP16 of the chimpanzee displays more than 98% nucleotide sequence identity with the human CASP16P gene but, like CASP16 genes of other primates, has an intact protein coding sequence. We conclude that caspase-16 structurally differs from other mammalian caspases, and the pseudogenization of CASP16 distinguishes humans from their phylogenetically closest relatives.
Keywords: Caspase; Evolution; Protein domain; Pseudogenization; Pyroptosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest. Ethical Approval: Not applicable. No procedures requiring ethics approval were performed in this study. Consent to Participate: Not applicable. Consent to Publish: Not applicable.
Figures



References
-
- Akaike H (1998) Information theory and an extension of the maximum likelihood principle. In: Parzen E et al (eds) Selected Papers of Hirotugu Akaike. Springer, New York, pp 199–213
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

