Unraveling the immunomodulatory role of TIM-3 in head and neck squamous cell carcinoma: implications for targeted therapy
- PMID: 40392355
- PMCID: PMC12092856
- DOI: 10.1007/s12672-025-02673-2
Unraveling the immunomodulatory role of TIM-3 in head and neck squamous cell carcinoma: implications for targeted therapy
Abstract
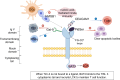
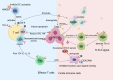
Head and neck squamous cell carcinoma (HNSCC) ranks among the most prevalent cancers globally, and despite improvements in treatment options such as surgery and radiotherapy, its survival rate remains low. With increased research in immunotherapy, antibodies against various immune checkpoints like programmed death receptor 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have been shown to be effective against a wide range of tumors. Nonetheless, survival benefits gained by HNSCC patients remain limited. T-cell immunoglobulin mucin-3 (TIM-3), an emerging immune checkpoint molecule, is found to be expressed in HNSCC and is involved in shaping the tumor immune microenvironment (TIME). TIM-3 is significant in the initiation and progression of HNSCC by modulating effector T cells, innate immune cells, and other components of the immune system. Inhibiting TIM-3 can restore T cell function and enhance the immune response against HNSCC, making it a promising immunotherapeutic target for this disease. This article reviews the expression of TIM-3 in HNSCC and its immunomodulatory mechanism and briefly introduces the combined application and development prospects of TIM-3 as a potential immunotherapeutic target.
Keywords: Combination immunotherapy; Head and neck squamous cell carcinoma; Human papillomavirus; T-cell immunoglobulin mucin-3; Tumor immune microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Novel Effector Phenotype of Tim-3+ Regulatory T Cells Leads to Enhanced Suppressive Function in Head and Neck Cancer Patients.Clin Cancer Res. 2018 Sep 15;24(18):4529-4538. doi: 10.1158/1078-0432.CCR-17-1350. Epub 2018 Apr 30. Clin Cancer Res. 2018. PMID: 29712685 Free PMC article.
-
The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer.Phytomedicine. 2021 Nov;92:153758. doi: 10.1016/j.phymed.2021.153758. Epub 2021 Sep 16. Phytomedicine. 2021. PMID: 34592487
-
Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma.Int J Oral Sci. 2020 May 28;12(1):16. doi: 10.1038/s41368-020-0084-8. Int J Oral Sci. 2020. PMID: 32461587 Free PMC article. Review.
-
The dynamic role of immune checkpoint molecules in diagnosis, prognosis, and treatment of head and neck cancers.Biomed Pharmacother. 2024 Feb;171:116095. doi: 10.1016/j.biopha.2023.116095. Epub 2024 Jan 6. Biomed Pharmacother. 2024. PMID: 38183744 Review.
-
Differential impact of TIM-3 ligands on NK cell function.J Immunother Cancer. 2025 Jan 7;13(1):e010618. doi: 10.1136/jitc-2024-010618. J Immunother Cancer. 2025. PMID: 39773563 Free PMC article.
References
-
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–96. 10.1016/j.mayocp.2015.12.017. - PubMed
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. 10.3322/caac.21834. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
