Distinct effects of mucin on phage-host interactions in model systems of beneficial and pathogenic bacteria
- PMID: 40392378
- PMCID: PMC12092537
- DOI: 10.1007/s00705-025-06322-5
Distinct effects of mucin on phage-host interactions in model systems of beneficial and pathogenic bacteria
Abstract
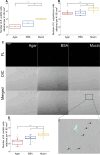
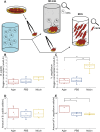
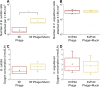
Phage-host interactions that occur in host-associated microbiomes are influenced by a plethora of environmental factors. Mucins are glycoproteins that represent the main component of mucus, which is found in the animal digestive tract and on the surface of certain organs, serving as the first line of defense against toxins and pathogens. Previous studies have shown that lytic phages have an important influence on the microbial composition in mucosal areas. Our study expands this knowledge to interactions between previously untested lytic phages targeting probiotic and pathogenic bacteria, as well as temperate phages targeting probiotic bacteria. These interactions could be important in shaping microbial communities and affecting the well-being of their host. This study demonstrates that mucins enhance the adherence of Vibrio anguillarum lytic phages and Bacillus subtilis lytic and temperate phages, as well as B. subtilis and V. anguillarum cells, to solid surfaces. Our results also show that mucins positively affect the attachment of B. subtilis cells even in the presence of phages. This positive effect was not observed in the case of V. anguillarum. This suggests that mucin may shield certain bacteria from phage infections. We also found that mucin influenced the metabolic activity of the two tested bacterial species differently, with strong positive effects on V. anguillarum but not on B. subtilis. This work supports previous findings that phages adhere efficiently to mucus and extends these studies to include other beneficial and pathogenic bacterial species. It also reveals that mucins have different effects on phage-host interactions in different phage-host systems, which may have implications for phage therapies or probiotic treatment strategies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Figures

Similar articles
-
Relevance of the bacteriophage adherence to mucus model for Pseudomonas aeruginosa phages.Microbiol Spectr. 2024 Aug 6;12(8):e0352023. doi: 10.1128/spectrum.03520-23. Epub 2024 Jun 24. Microbiol Spectr. 2024. PMID: 38912817 Free PMC article.
-
Bacteriophage Adherence to Mucus Mediates Preventive Protection against Pathogenic Bacteria.mBio. 2019 Nov 19;10(6):e01984-19. doi: 10.1128/mBio.01984-19. mBio. 2019. PMID: 31744913 Free PMC article.
-
Stumbling across the Same Phage: Comparative Genomics of Widespread Temperate Phages Infecting the Fish Pathogen Vibrio anguillarum.Viruses. 2017 May 20;9(5):122. doi: 10.3390/v9050122. Viruses. 2017. PMID: 28531104 Free PMC article.
-
Phage transmission strategies: are phages farming their host?Curr Opin Microbiol. 2024 Jun;79:102481. doi: 10.1016/j.mib.2024.102481. Epub 2024 Apr 26. Curr Opin Microbiol. 2024. PMID: 38677076 Review.
-
Diversity of phage infection types and associated terminology: the problem with 'Lytic or lysogenic'.FEMS Microbiol Lett. 2016 Apr;363(7):fnw047. doi: 10.1093/femsle/fnw047. Epub 2016 Feb 29. FEMS Microbiol Lett. 2016. PMID: 26925588 Review.
References
-
- Mäkelä K, Laanto E, Sundberg L-R (2024) Determinants in the phage life cycle: the dynamic nature of ssDNA phage FLiP and host interactions under varying environmental conditions and growth phases. Environ Microbiol 26:e16670. 10.1111/1462-2920.16670 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

