Three-dimensional culture in a bioengineered matrix and somatic cell complementation to improve growth and survival of bovine preantral follicles
- PMID: 40392485
- PMCID: PMC12167402
- DOI: 10.1007/s10815-025-03497-3
Three-dimensional culture in a bioengineered matrix and somatic cell complementation to improve growth and survival of bovine preantral follicles
Abstract
Purpose: Here, we explored poly(ethylene glycol) (PEG) bioengineered hydrogels for bovine preantral follicle culture with or without ovarian cell co-culture and examined the potential for differentiation of bovine embryonic stem cells (bESCs) towards gonadal somatic cells to develop a system better mimicking the ovarian microenvironment.
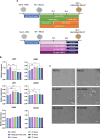
Methods: Bovine preantral follicles were first cultured in two-dimensional (2D) control or within PEG hydrogels (3D) and then co-cultured within PEG hydrogels with bovine ovarian cells (BOCs) to determine growth and viability. Finally, we tested conditions to drive differentiation of bESCs towards the intermediate mesoderm and bipotential gonad fate.
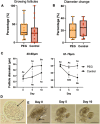
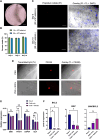
Results: Primary follicles grew over the 10-day culture period in PEG hydrogels compared to 2D control. Early secondary follicles maintained a similar diameter within the PEG while control follicles decreased in size. Follicles lost viability after co-encapsulation with BOCs; BOCs lost stromal cell signature over the culture period within hydrogels. Induction of bESCs towards gonadal somatic fate under WNT signaling was sufficient to upregulate intermediate mesoderm (LHX1) and early coelomic epithelium/bipotential gonad markers (OSR1, GATA4, WT1). Higher BMP4 concentrations upregulated the lateral plate mesoderm marker FOXF1. PAX3 expression was not induced, indicating absence of the paraxial mesoderm lineage.
Conclusions: Culture of primary stage preantral follicles in PEG hydrogels promoted growth compared to controls; BOCs did not maintain identity in the PEG hydrogels. Collectively, we demonstrate that PEG hydrogels can be a potential culture system for early preantral follicles pending refinements, which could include addition of ESC-derived ovarian somatic cells using the protocol described here.
Keywords: Biomimetic; Bovine; Embryonic stem cells; Ovary; Preantral follicle; Three-dimensional culture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Update of
-
Three-dimensional culture in a bioengineered matrix and somatic cell complementation to improve growth and survival of bovine preantral follicles.bioRxiv [Preprint]. 2024 Aug 7:2024.07.18.604061. doi: 10.1101/2024.07.18.604061. bioRxiv. 2024. Update in: J Assist Reprod Genet. 2025 May;42(5):1509-1523. doi: 10.1007/s10815-025-03497-3. PMID: 39071337 Free PMC article. Updated. Preprint.
References
-
- Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadão AJ, Plancha CE. Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev. 1999;54:163–72. 10.1002/(SICI)1098-2795(199910)54:2<163::AID-MRD8>3.0.CO;2-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

