Pericytes in hematogenous metastasis: mechanistic insights and therapeutic approaches
- PMID: 40392500
- PMCID: PMC12238105
- DOI: 10.1007/s13402-025-01073-6
Pericytes in hematogenous metastasis: mechanistic insights and therapeutic approaches
Abstract
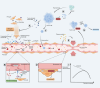
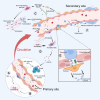
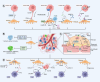
Metastasis, the leading cause of cancer-related deaths, underscores the critical need to understand its regulatory mechanisms to improve prevention and treatment strategies for late-stage tumors. Hematogenous dissemination is a key route of metastasis. However, as the gatekeeper of vessels, the role of pericytes in hematogenous metastasis remains largely unknown. In this review, we comprehensively explore the contributions of pericytes throughout the metastatic cascade, particularly their functions that extend beyond influencing tumor angiogenesis. Pericytes should not be perceived as passive bystanders, but rather as active participants in various stages of the metastatic cascade. Pericytes-targeted therapy may provide novel insights for preventing and treating advanced-stage tumor.
Keywords: Hematogenous metastasis; Pericytes; Plasticity; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Molecular-targeted first-line therapy for advanced gastric cancer.Cochrane Database Syst Rev. 2016 Jul 19;7(7):CD011461. doi: 10.1002/14651858.CD011461.pub2. Cochrane Database Syst Rev. 2016. PMID: 27432490 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
Publication types
MeSH terms
Grants and funding
- 2021JH2/10300053/Livelihood Science and Technology Project of Liaoning Province
- JYTMS20230101/Basic Scientific Research Project of Education Department of Liaoning Province
- 81974377/Natural Science Foundation of China
- 2023JH2/20200128/Funding Project of Supporting the High-Quality Development of China Medical University By Department of Science and Technology of Liaoning Province
LinkOut - more resources
Full Text Sources
Medical

