Mastocytosis in the Skin: Approach to Diagnosis, Evaluation, and Management in Adult and Pediatric Patients
- PMID: 40392511
- PMCID: PMC12325572
- DOI: 10.1007/s40257-025-00947-7
Mastocytosis in the Skin: Approach to Diagnosis, Evaluation, and Management in Adult and Pediatric Patients
Erratum in
-
Correction to: Mastocytosis in the Skin: Approach to Diagnosis, Evaluation, and Management in Adult and Pediatric Patients.Am J Clin Dermatol. 2025 Jul 23. doi: 10.1007/s40257-025-00972-6. Online ahead of print. Am J Clin Dermatol. 2025. PMID: 40702396 No abstract available.
Abstract
Mastocytosis is characterized by the clonal infiltration and proliferation of neoplastic mast cells into target organs. Clinical features of mastocytosis are based in large part on dysregulated mast cell mediator release. Affected individuals may present with isolated skin involvement or multisystemic disease with a spectrum of symptoms including anaphylaxis, pathologic fractures, and chronic gastrointestinal, neurocognitive, musculoskeletal, and constitutional symptoms. The term "mastocytosis in the skin" refers to individuals with cutaneous infiltration and encompasses both localized and systemic forms of disease. Cutaneous involvement is further categorized into cutaneous mastocytoma, diffuse cutaneous mastocytosis, and maculopapular cutaneous mastocytosis based on morphology. In ~95% of patients with systemic mastocytosis, the disease is driven by the KIT D816V somatic variant. The aim of this clinical review is to highlight the diagnostic considerations, management complexities, and evolving treatment landscape that must be considered when evaluating a patient presenting with mastocytosis in their skin. Clinical manifestations, histopathology, and laboratory parameters are essential to diagnosis and determining the disease burden in those with known or suspected systemic mastocytosis. Once appropriately staged, both skin-directed therapy as well as novel systemic treatment options, including selective tyrosine kinase inhibitors, can be considered with the potential to improve patient outcomes.
© 2025. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Declarations. Funding: Blueprint Medicines Corporation provided funding for the preparation of this article. Conflicts of Interest: Lauren M. Madigan served on an international advisory board for Blueprint Medicines and participated as a speaker in a non-Continuing Medical Education (CME) session on recognizing and diagnosis mastocytosis; Section editor, JAMA Dermatology. Nathan A. Boggs participated in a Blueprint Medicines-sponsored patient advocacy summit; participated in developing a Blueprint-sponsored webinar for the College of American Pathologists. Anton V. Rets participated in developing a Blueprint Medicines-sponsored webinar for the College of American Pathologists. Alejandro A. Gru is an investigator for Innate Pharma, Stemline Therapeutics, and Dren Bio; a consultant for Blueprint Medicines; and a speaker for Kyowa Kirin. Tsewang Tashi is a member of the advisory board and a primary investigator in Blueprint Medicines clinical trials, Cogent Biosciences, and PharmaEssentia; a member of the study steering committee for Blueprint Medicines clinical trials. David. A Wada and Melody C. Carter have no conflicts of interest that are directly relevant to the content of this article. Scott R. Florell is a consultant for Orlucent Inc. Ethics Approval: Not applicable. Consent to Participate: Not applicable. Consent for Publication: Patients or their representatives provided consent for the publication of any identifiable photographs. Availability of Data and Material: Not applicable. Code Availability: Not applicable. Authors’ Contributions: All authors contributed to the concept, research, and writing of the manuscript. All authors read and approved the final manuscript.
Figures

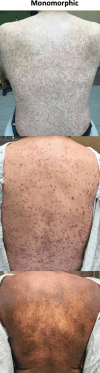
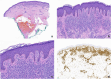
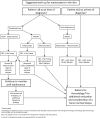
Similar articles
-
Characteristics and Therapeutic Strategies for Diffuse Cutaneous Mastocytosis.JAMA Dermatol. 2025 Aug 1;161(8):855-862. doi: 10.1001/jamadermatol.2025.1488. JAMA Dermatol. 2025. PMID: 40434754
-
Systemic mastocytosis--a systematic review.Dan Med J. 2012 Mar;59(3):A4397. Dan Med J. 2012. PMID: 22381091
-
Clinical and Biological Characteristics of Four Patients with Aggressive Systemic Mastocytosis Treated with Midostaurin.Biomedicines. 2025 Jul 7;13(7):1655. doi: 10.3390/biomedicines13071655. Biomedicines. 2025. PMID: 40722727 Free PMC article.
-
AGA Clinical Practice Update on GI Manifestations and Autonomic or Immune Dysfunction in Hypermobile Ehlers-Danlos Syndrome: Expert Review.Clin Gastroenterol Hepatol. 2025 Jul;23(8):1291-1302. doi: 10.1016/j.cgh.2025.02.015. Epub 2025 May 19. Clin Gastroenterol Hepatol. 2025. PMID: 40387691 Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Cohen SS, Skovbo S, Vetergaard H, et al. Epidemiology of systemic mastocytosis in Denmark. Br J Haematol. 2014;166(4):521–8. - PubMed
-
- Valent P, Sperr WR, Schwartz LB, et al. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114(1):3–11. - PubMed
-
- Brockow K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol Allergy Clin North Am. 2014;34(2):283–95. - PubMed
-
- Carter MC, Metcalfe DD. Mastocytosis. In: Young NS, Gerson SL, Hight KH, editors. Clinical hematology. Philadelphia (PA): Mosby, Inc.; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

