CDKN2A/B status versus morphology in diagnosing WHO grade 4 IDH-mutated astrocytomas: what is the clinical relevance?
- PMID: 40392514
- PMCID: PMC12208962
- DOI: 10.1007/s11060-025-05078-x
CDKN2A/B status versus morphology in diagnosing WHO grade 4 IDH-mutated astrocytomas: what is the clinical relevance?
Abstract
Purpose: In the 2021 WHO classification system for central nervous system tumors, the diffuse glioma subgroup IDH-mutated (IDHm) astrocytomas WHO grade 4 was introduced. The diagnosis can be based upon molecular or histopathological morphological criteria. Here we explore whether phenotype and survival of IDHm astrocytomas WHO grade 4 differed across the criteria used for diagnosis.
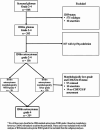
Methods: Patients with IDHm astrocytoma, WHO grade 4, were included from Sahlgrenska University Hospital and TCGA database. We created three subgroups based upon the criteria for diagnosis of WHO grade 4; (1) homozygous CDKN2A/B deletion; (2) morphological (necrosis and/or microvascular proliferation); (3) combined subgroup with both homozygous CDKN2A/B deletion and morphological grade 4 criteria.
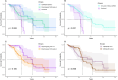
Results: We included 90 patients (local cohort, n = 35, TCGA cohort, n = 55) with IDHm astrocytoma, WHO grade 4. The median survival was 4.1 years (95% CI 3.0-5.3). Survival was comparable when the diagnosis was based on homozygous CDKN2A/B deletion and on morphological WHO grade 4 criteria (5.2 vs. 5.3 years). However, in the combined subgroup, survival was significantly shorter (2.8 years, p = 0.006).
Conclusion: The different subgroups of IDHm astrocytoma WHO grade 4 share similar characteristics. Patients whose tumors exhibit combined criteria have worse prognosis.
Keywords: CDKN2A/B; DNA methylation; IDH-mutant astrocytoma; WHO grade 4.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: An approval by the Swedish ethical review authority (with Dnr-number 2022-00160-01) was received prior to this study. Due to the retrospective nature of the study, a waiver from the need for informed consent was provided. Consent to participate: All patients in the following study were retrospectively included and the ethical committee waived the need of informed consent for these patients. Competing interests: The authors declare no competing interests.
Figures

References
-
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. 10.1093/neuonc/noab106 - PMC - PubMed
-
- Appay R, Dehais C, Maurage CA, Alentorn A, Carpentier C, Colin C, Ducray F, Escande F, Idbaih A, Kamoun A, Marie Y, Mokhtari K, Tabouret E, Trabelsi N, Uro-Coste E, Delattre JY, Figarella-Branger D, Network P (2019) CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol 21:1519–1528. 10.1093/neuonc/noz124 - PMC - PubMed
-
- Dipasquale A, Franceschi E, Giordano L, Maccari M, Barigazzi C, Di Nunno V, Losurdo A, Persico P, Di Muzio A, Navarria P, Pessina F, Padovan M, Santoro A, Lombardi G, Simonelli M (2024) Dissecting the prognostic signature of patients with Astrocytoma isocitrate dehydrogenase-mutant grade 4: a large multicenter, retrospective study. ESMO Open 9:103485. 10.1016/j.esmoop.2024.103485 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

