Real-world evidence from Japan regarding survival outcomes and treatment sequence in patients receiving CDK4/6 inhibitor plus endocrine therapy as first- or second-line treatment for hormone receptor-positive, HER2-negative advanced or metastatic breast cancer
- PMID: 40392524
- PMCID: PMC12174191
- DOI: 10.1007/s12282-025-01713-7
Real-world evidence from Japan regarding survival outcomes and treatment sequence in patients receiving CDK4/6 inhibitor plus endocrine therapy as first- or second-line treatment for hormone receptor-positive, HER2-negative advanced or metastatic breast cancer
Erratum in
-
Correction: Real‑world evidence from Japan regarding survival outcomes and treatment sequence in patients receiving CDK4/6 inhibitor plus endocrine therapy as first‑ or second‑line treatment for hormone receptor-positive, HER2‑negative advanced or metastatic breast cancer.Breast Cancer. 2025 Sep;32(5):1157-1158. doi: 10.1007/s12282-025-01736-0. Breast Cancer. 2025. PMID: 40549070 Free PMC article. No abstract available.
Abstract
Background: A cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) plus endocrine therapy (ET) is a current standard first-/second-line treatment for hormone receptor (HR)-positive, HER2-negative advanced/metastatic breast cancer (AMBC). We aimed to provide real-world evidence regarding CDK4/6i therapy in this population.
Methods: In this multicenter observational study, data from patients who had started CDK4/6i therapy between January 1, 2019, and December 31, 2021, as first-/second-line treatment for AMBC were used; real-world progression-free survival (rwPFS), chemotherapy-free survival, and overall survival were analyzed using the Kaplan-Meier method. Additionally, data were analyzed by separating patients with treatment-free interval (TFI) < 12 months (deemed resistant to ET) from the first-line treatment group (hereafter, the exclusive first-line treatment group).
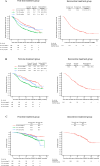
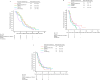
Results: Data from 745 patients were analyzed. Compared with palbociclib, abemaciclib was used in younger patients and those with expected poor prognosis. Median rwPFS was 36.8, 17.8, and 31.4 months in patients with de novo stage IV disease, TFI < 12 months, and TFI ≥ 12 months, respectively, in the first-line treatment group, and 17.4 months in the second-line treatment group. In the exclusive first-line treatment group, median rwPFS of the subsequent treatment after initial CDK4/6i plus ET was < 7 months, regardless of the type of subsequent treatment; prognosis was especially poor in those who were switched to chemotherapy.
Conclusions: The real-world survival outcomes found in this study for patients receiving first-/second-line CDK4/6i therapy were consistent with those of randomized phase 3 studies. As outcomes of subsequent treatment after initial CDK4/6i plus ET remain insufficient, further improvement in treatment is necessary.
Keywords: Abemaciclib; Advanced or metastatic breast cancer; Cyclin-dependent kinase 4/6 inhibitor; Palbociclib; Real-world evidence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: TY reports receiving payment/honoraria from Eli Lilly Japan, AstraZeneca, Chugai Pharmaceutical, Kyowa Kirin, Pfizer, MSD, Eisai, and Novartis Pharma. YT reports grant/contract from Eli Lilly; receiving payment/honoraria from Eli Lilly Japan, Pfizer, Chugai Pharmaceutical, Daiichi Sankyo, MSD, and AstraZeneca. YO reports receiving honoraria from Daiichi Sankyo, Pfizer, Eli Lilly Japan, and Kyowa Kirin. KW reports receiving payment/honoraria from Chugai Pharmaceutical, Eli Lilly Japan, Nippon Kayaku, Kyowa Kirin, Novartis Pharma, Taiho Pharmaceutical, Eisai, Pfizer, Shionogi Pharmaceutical, Daiichi Sankyo, and AstraZeneca. SEN reports receiving payment/honoraria from Eli Lilly Japan, Pfizer, Daiichi Sankyo, Chugai Pharmaceutical, Eisai, MSD, Gilead Sciences, and Kyowa Kirin; participation on a Data Safety Monitoring Board or Advisory Board for Daiichi Sankyo and Chugai Pharmaceutical. NS (Shibata) reports research grants to institution from Daiichi Sankyo, AstraZeneca, and MSD; receiving consulting fees from Kyowa Kirin; receiving lecture honoraria from Kyowa Kirin, MSD, Daiichi Sankyo, Chugai Pharmaceutical, Pfizer, Eisai, Yakult, Taiho Pharmaceutical, Eli Lilly Japan, Nippon Kayaku, Merck Biopharma, and Bristol Myers Squibb; participation on an Advisory Board for Kyowa Kirin and Daiichi Sankyo. CO reports receiving payment/honoraria from Eli Lilly Japan. HB reports receiving payment/honoraria from Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, Eisai, Kyowa Kirin, AstraZeneca, Pfizer, and MSD. NT (Tsunoda) reports receiving payment/honoraria from Eli Lilly Japan, Pfizer, Chugai Pharmaceutical, AstraZeneca, and Eisai. KK reports receiving payment/honoraria from Pfizer and Chugai Pharmaceutical. MT (Takada) reports grants to institution from Yakult and Guardant Health Japan; receiving payment/honoraria from Daiichi Sankyo, AstraZeneca, Taiho Pharmaceutical, Eli Lilly Japan, MSD, Pfizer, Eisai, Chugai Pharmaceutical, Devicor Medical Japan, and Mitaka Kohki. NT (Toriguchi), NS (Sekine), and TK report being employees of Eli Lilly Japan and minor shareholders of Eli Lilly and Company. SS reports grants to institution from Taiho Pharmaceutical, Eisai, Takeda Pharmaceutical, Chugai Pharmaceutical, and Daiichi Sankyo; contracted clinical trials for MSD, AstraZeneca, Gilead Sciences, Eli Lilly Japan, Sanofi, Chugai Pharmaceutical, and Daiichi Sankyo; receiving payment/honoraria from Chugai Pharmaceutical, Kyowa Kirin, MSD, Novartis Pharma, Eisai, Takeda Pharmaceutical, Daiichi Sankyo, Eli Lilly Japan, AstraZeneca, Pfizer, Taiho Pharmaceutical, Ono Pharmaceutical, Nippon Kayaku, Gilead Sciences, and Exact Sciences; participation on a Data Safety Monitoring Board or Advisory Board for Chugai/Roche, AstraZeneca, Eli Lilly Japan, Pfizer, Kyowa Kirin, Daiichi Sankyo, and MSD; being an executive board member for Japan Breast Cancer Research Group (JBCRG), Japanese Breast Cancer Society (JBCS), Japanese Society of Medical Oncology (JSMO), and Breast International Group (BIG). YS reports receiving payment/honoraria from Pfizer, Daiichi Sankyo, Eli Lilly Japan, MSD, Kyowa Kirin, Celltrion Healthcare Japan, AstraZeneca, Eisai, Chugai Pharmaceutical, Nippon Kayaku, and Sysmex. SM reports grants to institution from Eisai; receiving payment/honoraria from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan, MSD, and Ono Pharmaceutical. NM grants to institution from Chugai Pharmaceutical, Eli Lilly Japan, AstraZeneca, Pfizer, Daiichi Sankyo, MSD, Eisai, Novartis Pharma, Gilead Sciences, and Ono Pharmaceutical; receiving payment/honoraria from Chugai Pharmaceutical, Pfizer, AstraZeneca, Eli Lilly Japan, Daiichi Sankyo, and Eisai; being a representative/member of Board of Directors (unpaid) for JBCRG, JBCS, Japan Society of Clinical Oncology (JSCO), and Japan Association of Breast Cancer Screening (JABCS). YK, MY, MT (Tsukabe), FF, and KY report no conflict of interest regarding this manuscript. Research involving human participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice (ICH-GCP) Guideline, and Ethical Guidelines for Clinical Research of the Ministry of Health, Labour and Welfare of Japan. The protocol [no. 23136 (T34)] was approved by the institutional review board of Osaka University Hospital (Osaka, Japan). Informed consent: Patients provided consent to the use of their data by either providing written informed consent or on an opt-out basis.
Figures


References
-
- Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–14. 10.1007/s10549-013-2711-y. - PubMed
-
- Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39(35):3959–80. 10.1200/JCO.21.01392. - PMC - PubMed
-
- European Society for Medical Oncology. ESMO clinical practice guidelines: breast cancer. https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practi....
-
- Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, PALOMA3 Study Group, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–19. 10.1056/NEJMoa1505270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

