Deescalation From Ticagrelor to Clopidogrel for Myocardial Infarction With Chronic Kidney Disease: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 40392554
- PMCID: PMC12093190
- DOI: 10.1001/jamanetworkopen.2025.11297
Deescalation From Ticagrelor to Clopidogrel for Myocardial Infarction With Chronic Kidney Disease: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Chronic kidney disease (CKD) is a significant risk factor for both ischemic and bleeding complications following percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI). Optimizing dual antiplatelet therapy (DAPT) is essential for improving clinical outcomes.
Objective: To evaluate whether an 11-month, unguided deescalation strategy from ticagrelor to clopidogrel was associated with reduced bleeding without an increase in ischemic events in stabilized patients with CKD after AMI.
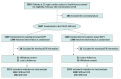
Design, setting, and participants: This was a post hoc secondary analysis of the multicenter, open-label, Ticagrelor vs Clopidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-AMI) randomized clinical trial conducted at 32 major cardiac centers in South Korea. Patients with biomarker-positive AMI who tolerated 1 month of ticagrelor-based DAPT after PCI were included in the trial. Patient enrollment occurred from February 2014 to December 2018, with follow-up at 30 days and 3, 6, and 12 months after PCI. The present analysis focused on the subgroup of patients with CKD (estimated glomerular filtration rate [eGFR]<60 mL/min/1.73 m2). Data analyses were performed from July 2023 to October 2024.
Interventions: In the TALOS-AMI trial, patients were randomized 1:1 to continue ticagrelor (active control group) or switch to clopidogrel (deescalation group) for 11 months after PCI.
Main outcomes and measures: The primary end point was a composite of death from cardiovascular disease, myocardial infarction, stroke, and bleeding (Bleeding Academic Research Consortium [BARC] types 2, 3, or 5).
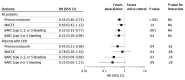
Results: Of 2646 patients included in the trial, 305 had CKD (11.5%; mean [SD] age, 67.2 [11.4] years; 223 males [73.1%]; mean [SD] eGFR, 49.7 [9.5] mL/min/1.73 m2) and 2341 did not have CKD (1975 male [84.4%]; mean [SD] age, 59.0 [11.0] years). Patients with CKD had an increased risk of ischemic events compared with patients without CKD (hazard ratio [HR], 2.47; 95% CI, 1.38-4.42; P = .002), but there was no difference in bleeding risk (HR, 1.36; 95% CI, 0.80-2.31; P = .26). Among patients with CKD, deescalation (n = 160) vs active control (n = 145) was associated with reduced risks of the primary end point (10 patients [6.2%] vs 19 [13.1%]; HR, 0.45 [95% CI, 0.21-0.98]; P = .04) and BARC 2, 3, or 5 bleeding (4 patients [2.5%] vs 12 [8.3%]; HR, 0.29 [95% CI, 0.09-0.89]; P = .03). No increased risk of ischemic events was observed following deescalation (7 patients [4.4%] vs 8 [5.5%]; HR, 0.78 [95% CI, 0.28-2.16]; P = .64).
Conclusions and relevance: In this secondary analysis of a randomized clinical trial, deescalation from ticagrelor to clopidogrel at 1 month after PCI for AMI was associated with significant reduction in bleeding without increased risk of ischemic events among study-eligible patients with CKD. Further adequately powered studies are needed to validate these findings.
Conflict of interest statement
Figures

References
-
- Lawton JS, Tamis-Holland JE, Bangalore S, et al. . 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e4-e17. doi:10.1161/CIR.0000000000001039 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

