Human mesofluidic intestinal model for studying transport of drug carriers and bacteria through a live mucosal barrier
- PMID: 40392585
- PMCID: PMC12091270
- DOI: 10.1039/d4lc00774c
Human mesofluidic intestinal model for studying transport of drug carriers and bacteria through a live mucosal barrier
Abstract
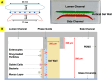
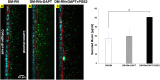
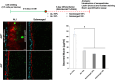
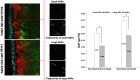
The intestinal mucosal barrier forms a critical interface between lumen contents such as bacteria, drugs, and drug carriers and the underlying tissue. Current in vitro intestinal models, while recapitulating certain aspects of this barrier, generally present challenges with respect to imaging transport across mucus and uptake into enterocytes. A human mesofluidic small intestinal chip was designed to enable facile visualization of a mucosal interface created by growing primary human intestinal cells on a vertical hydrogel wall separating channels representing the intestinal lumen and circulatory flow. Type I collagen, fortified via cross-linking to prevent deformation and leaking during culture, was identified as a suitable gel wall material for supporting primary organoid-derived human duodenal epithelial cell attachment and monolayer formation. Addition of DAPT and PGE2 to culture medium paired with air-liquid interface culture increased the thickness of the mucus layer on epithelium grown within the device for 5 days from approximately 5 μm to 50 μm, making the model suitable for revealing intriguing features of interactions between luminal contents and the mucus barrier using live cell imaging. Time-lapse imaging of nanoparticle diffusion within mucus revealed a zone adjacent to the epithelium largely devoid of nanoparticles up to 4.5 h after introduction to the lumen channel, as well as pockets of dimly lectin-stained mucus within which particles freely diffused, and apparent clumping of particles by mucus components. Multiple particle tracking conducted on the intact mucus layer in the chip revealed significant size-dependent differences in measured diffusion coefficients. E. coli introduced to the lumen channel were freely mobile within the mucus layer and appeared to intermittently contact the epithelial surface over 30 minute periods of culture. Mucus shedding into the lumen and turnover of mucus components within cells were visualized. Taken together, this system represents a powerful tool for visualization of interactions between luminal contents and an intact live mucosal barrier.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be viewed as potential competing interests: AbbVie funded the study, and AbbVie together with other authors' institutions jointly participated in study design, research, analysis and interpretation of data, writing, reviewing, and approving the publication, HSO and DL are employees of AbbVie and may own AbbVie stock. All other authors have no additional conflicts of interest to report.
Figures


Update of
-
Human Mesofluidic Intestinal Model for Studying Transport of Drug Carriers and Bacteria Through a Live Mucosal Barrier.bioRxiv [Preprint]. 2024 Sep 22:2024.09.18.613692. doi: 10.1101/2024.09.18.613692. bioRxiv. 2024. Update in: Lab Chip. 2025 Jun 10;25(12):2990-3004. doi: 10.1039/d4lc00774c. PMID: 39345622 Free PMC article. Updated. Preprint.
Similar articles
-
Human Mesofluidic Intestinal Model for Studying Transport of Drug Carriers and Bacteria Through a Live Mucosal Barrier.bioRxiv [Preprint]. 2024 Sep 22:2024.09.18.613692. doi: 10.1101/2024.09.18.613692. bioRxiv. 2024. Update in: Lab Chip. 2025 Jun 10;25(12):2990-3004. doi: 10.1039/d4lc00774c. PMID: 39345622 Free PMC article. Updated. Preprint.
-
Uptake of silica particulate drug carriers in an intestine-on-a-chip: towards a better in vitro model of nanoparticulate carrier and mucus interactions.Biomater Sci. 2019 May 28;7(6):2410-2420. doi: 10.1039/c9bm00058e. Biomater Sci. 2019. PMID: 30920576
-
Native gastrointestinal mucus: Critical features and techniques for studying interactions with drugs, drug carriers, and bacteria.Adv Drug Deliv Rev. 2023 Sep;200:114966. doi: 10.1016/j.addr.2023.114966. Epub 2023 Jun 15. Adv Drug Deliv Rev. 2023. PMID: 37329985 Free PMC article. Review.
-
Development of a Caco-2-based intestinal mucosal model to study intestinal barrier properties and bacteria-mucus interactions.Gut Microbes. 2025 Dec;17(1):2434685. doi: 10.1080/19490976.2024.2434685. Epub 2024 Dec 23. Gut Microbes. 2025. PMID: 39714032 Free PMC article.
-
Development and Functional Properties of Intestinal Mucus Layer in Poultry.Front Immunol. 2021 Oct 4;12:745849. doi: 10.3389/fimmu.2021.745849. eCollection 2021. Front Immunol. 2021. PMID: 34671361 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

