Phospho-RPA2 predicts response to platinum and PARP inhibitors in homologous recombination-proficient ovarian cancer
- PMID: 40392598
- PMCID: PMC12208538
- DOI: 10.1172/JCI189511
Phospho-RPA2 predicts response to platinum and PARP inhibitors in homologous recombination-proficient ovarian cancer
Abstract
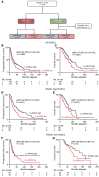
BACKGROUNDTreatment of tubo-ovarian high-grade serous carcinoma (HGSC) includes cytoreductive surgery, platinum-based chemotherapy, and often poly(ADP-ribose) polymerase (PARP) inhibitors. While homologous recombination (HR) deficiency is a well-established predictor of therapy sensitivity, over 50% of HR-proficient HGSCs also exhibit sensitivity. Currently, there are no biomarkers to identify which HR-proficient HGSCs will be sensitive to standard-of-care therapy. Replication stress may serve as a key determinant of response.METHODSWe evaluated phospho-RPA2-T21 (p-RPA2) foci via immunofluorescence as a biomarker of replication stress in formalin-fixed, paraffin-embedded HGSC samples collected at diagnosis from patients treated with platinum chemotherapy (discovery cohort, n = 31; validation cohort, n = 244) or PARP inhibitors (n = 63). Recurrent HGSCs (n = 38) were also analyzed. p-RPA2 score was calculated using automated imaging analysis.RESULTSSamples were defined as p-RPA2-high if more than 16% of cells had ≥2 p-RPA2 foci on automated analysis. In the discovery cohort, HR-proficient, p-RPA2-high HGSCs demonstrated significantly higher rates of a chemotherapy response score of 3 to platinum chemotherapy than HR-proficient, p-RPA2-low HGSCs. In the validation cohort, patients with HR-proficient, p-RPA2-high HGSCs had significantly longer survival after platinum treatment than those with HR-proficient, p-RPA2-low HGSCs. Additionally, the p-RPA2 assay effectively predicted survival outcomes in patients treated with PARP inhibitors and in recurrent HGSC samples.CONCLUSIONOur study underscores the importance of considering replication stress markers, such as p-RPA2, alongside HR status in therapeutic planning. This approach has the potential to increase the number of patients receiving effective therapy while reducing unnecessary toxicity.FUNDINGThe Reproductive Scientist Development Program, GOG Foundation, Pilot Translational and Clinical Studies function of the Washington University Institute of Clinical and Translational Sciences, the Foundation for Barnes-Jewish Hospital, Washington University School of Medicine Dean's Scholar Program, The Cancer Biology Pathway Training Grant (5T32CA113275-17), The Lucy, Anarcha, and Betsey (L.A.B.) Award from the Department of Obstetrics and Gynecology at Washington University School of Medicine, and Veterans Affairs Office of Research and Development (I01BX006020).
Keywords: Clinical Research; DNA repair; Molecular diagnosis; Obstetrics/gynecology; Oncology.
Figures




Update of
-
Replication stress marker phospho-RPA2 predicts response to platinum and PARP inhibitors in homologous recombination-proficient ovarian cancer.bioRxiv [Preprint]. 2024 Nov 25:2024.11.21.624682. doi: 10.1101/2024.11.21.624682. bioRxiv. 2024. Update in: J Clin Invest. 2025 May 20;135(13):e189511. doi: 10.1172/JCI189511. PMID: 39651311 Free PMC article. Updated. Preprint.
References
-
- Fagotti A, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. - DOI - PubMed
-
- Onda T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

