Viral kinetics among persons living with HIV (PLWH) on Dolutegravir-based antiretroviral Regimen: A retrospective and prospective analysis from selected HIV clinics in Ghana
- PMID: 40392867
- PMCID: PMC12091835
- DOI: 10.1371/journal.pone.0324360
Viral kinetics among persons living with HIV (PLWH) on Dolutegravir-based antiretroviral Regimen: A retrospective and prospective analysis from selected HIV clinics in Ghana
Abstract
Background: Dolutegravir (DTG)-based antiretroviral therapy has demonstrated superior efficacy, tolerability, and durability when compared to other HIV treatment regimens. However, monitoring viral kinetics is critical for determining treatment efficacy and making sound judgments. The purpose of this study was to assess viral kinetics in people living with HIV (PLWH) on DTG-based ART and identify characteristics related to virologic response in the Cape Coast Metropolis, Ghana.
Methods: Among people living with HIV (PLWH) attending HIV clinics between January 2020 and December 2023, a prospective and retrospective analysis of viral kinetics and clinical data were carried out. Data on viral loads, clinical laboratory results, ART regimen, and sociodemographic data were gathered. Viral loads analysis was undertaken using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, v2.0. Univariate and multivariate analyses were carried out to assess the variables related to virologic response.
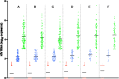
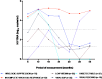
Results: Complete data was obtained for a total of 902 PLWH in this study. The average age was 45 ± 15.30 years, and 72.62% were female. The majority, 89.02% (835/902), had been on the DTG+3TC+TDF regimen. Over 60% had undetectable viral loads (<50 copies/mL). Univariate analysis shows a significant relationship between gender and virologic response, with females having a lower likelihood of virologic failure (OR: 0.60, 95% CI: 0.39-0.93, p-value = 0.024). In multivariate analysis, the duration of ART had various relationships with virologic response, with the odds ratio for two years reaching near significance (OR: 1.88, 95% CI: 0.98-3.59, p = 0.057). PLWH with viral loads >1000 copies/mL were 11.20% (101/902) while viral suppression, which was at detectable limits (>50 - ≤ 1000 cp/mL), was 13.08% (118/902) showing high rates of viral suppression.
Conclusion: The presence of virologic failures was of concern despite the high rates of viral suppression that DTG-based ART demonstrated. Undetectable viral suppression was higher than detectable viral suppression. Regular monitoring of viral kinetics, adherence, and comorbidities is essential to meeting the United Nations program on HIV/AIDS (UNAIDS) 95-95-95 targets and providing efficient therapeutic approaches for PLWH.
Copyright: © 2025 Badu Nyarko et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Virologically suppressed switch to Dolutegravir/Lamivudine 2-Drug regimen versus switch to commonly prescribed 3-Drug regimens in the United States.AIDS Res Ther. 2024 Oct 26;21(1):76. doi: 10.1186/s12981-024-00668-7. AIDS Res Ther. 2024. PMID: 39462377 Free PMC article.
-
Safety and efficacy of lamivudine/dolutegravir vs. bictegravir/emtricitabine/tenofovir alafenamide in antiretroviral-naive adults with HIV-1 infection in Shanghai, China: a single-centre retrospective study.J Med Microbiol. 2025 Jan;74(1). doi: 10.1099/jmm.0.001949. J Med Microbiol. 2025. PMID: 39773780
-
DTG + 3TC dual therapy for the treatment Naïve patients with viral load exceeding 500,000 copies/mL: a retrospective study.BMC Infect Dis. 2024 Jul 22;24(1):720. doi: 10.1186/s12879-024-09624-2. BMC Infect Dis. 2024. PMID: 39039487 Free PMC article.
-
Doing More With Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection.Ann Pharmacother. 2020 Dec;54(12):1252-1259. doi: 10.1177/1060028020933772. Epub 2020 Jun 9. Ann Pharmacother. 2020. PMID: 32517480 Review.
-
Prevalence of Emergent Dolutegravir Resistance Mutations in People Living with HIV: A Rapid Scoping Review.Viruses. 2024 Mar 4;16(3):399. doi: 10.3390/v16030399. Viruses. 2024. PMID: 38543764 Free PMC article.
References
-
- Clotet B, Feinberg J, van Lunzen J, Khuong-Josses M-A, Antinori A, Dumitru I, et al.. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31. doi: 10.1016/S0140-6736(14)60084-2 - DOI - PubMed
-
- Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB, et al.. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438–47. doi: 10.1016/S2352-3018(18)30132-2 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

