Does pre-emptive dexamethasone provide prophylaxis against sugammadex-induced bradycardia? A retrospective study
- PMID: 40392892
- PMCID: PMC12091743
- DOI: 10.1371/journal.pone.0323419
Does pre-emptive dexamethasone provide prophylaxis against sugammadex-induced bradycardia? A retrospective study
Abstract
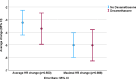
Sugammadex is a cyclodextrin used to reverse neuromuscular block with amino-steroid nondepolarizing muscle relaxants, rocuronium and vecuronium. Sugammadex-induced bradycardia was recently demonstrated in a single-blind, placebo-controlled study in patients receiving rocuronium for neuromuscular block. It has also been hypothesized that the bradycardia and rare instances of cardiac arrest occurring after the use of sugammadex may be due to a transient decrease in circulating corticosteroids, causing a temporary 'mini Addisonian crisis.' It was proposed that the administration of corticosteroids such as dexamethasone for post-operative nausea and vomiting (PONV) management might offer prophylaxis against these adverse occurrences. The study database was queried from a prospective study on sugammadex-related bradycardia, which was approved by the Human Studies Review Board and exempt from patient consent requirements. Patients were grouped into those that had or had not received dexamethasone as prophylaxis for PONV prior to the administration of sugammadex, and heart rate changes were evaluated 5 minutes after sugammadex administration. A total of 103 subjects were evaluated, of whom 38 received intravenous dexamethasone (either 4 mg, 8 mg, or 10 mg) during their anesthetic course and 65 patients had not received dexamethasone. The average heart rate (HR) slowing (3.2 bpm ± 3.9 in the control group, 3.7 bpm ± 3.8 in the dexamethasone group), and maximal HR slowing (5.0 bpm ± 3.9 in the control group, 5.0 bpm ± 3.8 in the dexamethasone group) over the five minutes following sugammadex administration were not significant between groups (average HR slowing p = 0.553, maximal HR slowing p = 0.988). These results potentially negate the proposed theory, or it may be that corticosteroids with more mineralocorticoid activity such as fludrocortisone or hydrocortisone are required to prevent this effect. Larger studies or prospective trials evaluating this effect with cortisol concentration measurement are needed to further evaluate the hypothesis.
Copyright: © 2025 Jahr et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
[Severe bradycardia and asystole associated with sugammadex: case report].Braz J Anesthesiol. 2019 Mar-Apr;69(2):218-221. doi: 10.1016/j.bjan.2018.09.010. Epub 2018 Oct 19. Braz J Anesthesiol. 2019. PMID: 30348442 Free PMC article.
-
Heart rate changes following the administration of sugammadex in children: a prospective, observational study.J Anesth. 2020 Apr;34(2):238-242. doi: 10.1007/s00540-019-02729-y. Epub 2020 Jan 24. J Anesth. 2020. PMID: 31980926
-
Dexamethasone Does Not Inhibit Sugammadex Reversal After Rocuronium-Induced Neuromuscular Block.Anesth Analg. 2016 Jun;122(6):1826-30. doi: 10.1213/ANE.0000000000001294. Anesth Analg. 2016. PMID: 27028777
-
The effect of dexamethasone on sugammadex reversal of rocuronium-induced neuromuscular blockade in surgical patients undergoing general anesthesia: A systematic review and meta-analysis.Medicine (Baltimore). 2021 Feb 5;100(5):e23992. doi: 10.1097/MD.0000000000023992. Medicine (Baltimore). 2021. PMID: 33592855 Free PMC article.
-
Clarifying the grey space of sugammadex induced bradycardia.Curr Opin Anaesthesiol. 2023 Aug 1;36(4):422-427. doi: 10.1097/ACO.0000000000001282. Epub 2023 Jun 12. Curr Opin Anaesthesiol. 2023. PMID: 37314178 Review.
References
-
- Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110:1020–5. - PubMed
-
- Merck & Co. Bridion (Sugammadex) Injection, for Intravenous Use: US Prescribing Information. 2015. [cited June 24, 2023]. Available from: http://www.accessdata.fda.gov/.
-
- Ebert TJ, Cumming CE, Roberts CJ, et al.. Characterizing the Heart Rate Effects from Administration of Sugammadex to Reverse Neuromuscular Blockade: An Observational Study in Patients. Anesth Analg. 2022;135(4):807–14. - PubMed
-
- Hirsch JG, Chia PA, Jahr JS. Sugammadex: A Review of the Considerations for Women of Childbearing Age. Am J Therap. 2023;30(20):e146–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources