Adverse events affecting recovery from seasonal influenza vaccination in the hypertensive population: A population-based pharmacovigilance analysis
- PMID: 40392910
- PMCID: PMC12091759
- DOI: 10.1371/journal.pone.0310474
Adverse events affecting recovery from seasonal influenza vaccination in the hypertensive population: A population-based pharmacovigilance analysis
Abstract
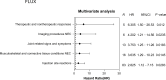
Seasonal influenza vaccination is crucial for preventing influenza and its complications. Data from the United States Vaccine Adverse Event Reporting System (VAERS) indicate a higher proportion of adverse events (AEs) after influenza vaccination in hypertensive people. However, there is limited evidence on AEs in hypertensive people following seasonal influenza vaccination. We identified 4647 individuals aged 18 years or older with a history of hypertension who received seasonal influenza vaccination and 6380 seasonal influenza-vaccine-induced AEs between 1 January 2013 and 23 June 2023 from VAERS. We identified two groups for comparison: recovery and no recovery from seasonal influenza-vaccine-induced AEs. Propensity score matching (PSM) was performed to adjust for potential confounding factors, including demographic characteristics (age, sex, and region) and season of onset. Cox regression analysis was used to calculate the risk ratio of reported adverse events (AEs) that affected recovery after seasonal influenza vaccination. Most AEs were nonserious and occurred within 48 hours. The most common AEs were general disorders and administration site conditions (therapeutic and non-therapeutic responses, inflammation) and musculoskeletal and connective tissue disorders (musculoskeletal and connective tissue pain and discomfort, bursal disorders, joint-related signs, and symptoms). All three types of seasonal influenza vaccines were associated with injection site reactions (47.07% trivalent influenza vaccine [TIA], hazard ratio, HR 2.04, 95% confidence interval, CI 1.22-3.40; 20.00% quadrivalent influenza vaccine [QIA], HR 2.81, 95% CI, 1.81-4.37; 67.48% influenza vaccine, unknown manufacturer [FLUX], HR 2.83, 95% CI, 1.12-7.15) and were the AEs affecting the largest proportion of delayed recoveries in the hypertensive population. Potential AEs following seasonal influenza vaccination may affect the recovery of the hypertensive population. The majority of AEs reported were general disorders, predominantly injection site reactions, and nonserious.
Copyright: © 2025 Wu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Post-licensure surveillance of trivalent adjuvanted influenza vaccine (aIIV3; Fluad), Vaccine Adverse Event Reporting System (VAERS), United States, July 2016-June 2018.Vaccine. 2019 Mar 7;37(11):1516-1520. doi: 10.1016/j.vaccine.2019.01.052. Epub 2019 Feb 7. Vaccine. 2019. PMID: 30739795
-
Safety Profile of GSK's Inactivated Quadrivalent Seasonal Influenza Vaccine in Belgium, Germany and Spain: Passive Enhanced Safety Surveillance Study for the 2019/2020 Influenza Season.Drug Saf. 2021 Dec;44(12):1375-1390. doi: 10.1007/s40264-021-01121-8. Epub 2021 Oct 25. Drug Saf. 2021. PMID: 34694589 Free PMC article.
-
Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the vaccine adverse event reporting system (VAERS) during July 1, 2021-June 30, 2022.Vaccine. 2023 Mar 10;41(11):1859-1863. doi: 10.1016/j.vaccine.2022.12.069. Epub 2023 Jan 9. Vaccine. 2023. PMID: 36669964 Free PMC article. Review.
-
An 8-Year Prospective, Observational, Multi-centre Post-Marketing Safety Surveillance Study Conducted in South Korea (2014-2022) Following the Introduction of GSK's Inactivated Quadrivalent Seasonal Influenza Vaccine (Fluarix Tetra) for Subjects Aged 6 Months and Older.Drug Saf. 2024 Apr;47(4):365-375. doi: 10.1007/s40264-024-01395-8. Epub 2024 Mar 14. Drug Saf. 2024. PMID: 38483767 Free PMC article.
-
Post-licensure surveillance of quadrivalent inactivated influenza (IIV4) vaccine in the United States, Vaccine Adverse Event Reporting System (VAERS), July 1, 2013-May 31, 2015.Vaccine. 2016 May 11;34(22):2507-12. doi: 10.1016/j.vaccine.2016.03.048. Epub 2016 Mar 23. Vaccine. 2016. PMID: 27015735 Free PMC article. Review.
References
-
- World Health Organization. Influenza (Seasonal). [cited 29 Aug 2024] https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

