Assessing Commercial Tissue-Based Assays for Autoimmune Neurologic Disorders (I): Antibodies to Intracellular Antigens
- PMID: 40393022
- PMCID: PMC12153943
- DOI: 10.1212/NXI.0000000000200410
Assessing Commercial Tissue-Based Assays for Autoimmune Neurologic Disorders (I): Antibodies to Intracellular Antigens
Abstract
Background and objectives: Current strategies to detect autoantibodies against intracellular neural antigens (IC-Abs) include tissue-based assays (TBAs) alongside line blots or cell-based assays (CBAs). Many clinical laboratories use commercially available TBAs as a screening test, but their diagnostic yield has not been assessed. We determined the performance of 2 commercial TBAs in detecting IC-Abs.
Methods: We analyzed samples from 100 patients with autoimmune or paraneoplastic neurologic syndromes harboring IC-Abs (confirmed by in-house TBAs and line blots or CBAs) and 50 negative controls. IC-Abs samples included serum (10 each for Hu, Yo, Ri, SOX1, CV2, Ma2, Tr, amphiphysin, and GAD65 antibodies) or CSF (10 with GFAP antibodies) samples. Two commercial indirect immunofluorescence (IIF) TBAs (INOVA and EUROIMMUN) were assessed by 2 experienced investigators and 3 less experienced raters, all blinded to antibody status. Discordant results were re-evaluated through interrater discussion and assessed using Cohen's kappa.
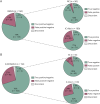
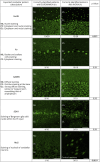
Results: The 2 experienced raters showed substantial agreement (85% for INOVA, 83% for EUROIMMUN) on negative/positive results, which increased to >95% after interrater discussion (Cohen's kappa 0.95 and 0.93, respectively). With IIF-INOVA, they correctly identified 118 of 150 samples (79%) and misclassified 28 of 150 (19%, 2 false positives and 26 false negatives) while results remained discordant in the remaining 4 of 150 samples (2%). With IIF-EUROIMMUN, they correctly identified 105 of 150 samples (70%) and misclassified 40 of 150 (27%, 6 false positives, 34 false negatives), with discordance in 5 of 150 samples (3%). Overall, the sensitivity was 73% for IIF-INOVA and 66% for IIF-EUROIMMUN. The specificity was 96% for IIF-INOVA and 88% for IIF-EUROIMMUN. Both TBAs showed low sensitivity in detecting CV2, SOX1, and amphiphysin antibodies while Ma2 antibodies were missed mainly by IIF-EUROIMMUN and Hu/Ri antibodies by IIF-INOVA. Antibody-specific immunostaining patterns were correctly identified in 62 of 100 positive samples with IIF-INOVA and 55 of 100 with IIF-EUROIMMUN (p = 0.34). Less experienced raters showed higher rates of false-positive results (up to 22% with IIF-EUROIMMUN).
Discussion: The performance of commercial IIF-TBAs for IC-Abs detection is suboptimal, exhibiting high false-negative rates of 25%-35%. Therefore, commercial TBAs should not be used alone for IC-Abs screening, but alongside more sensitive techniques, such as line blots. Discordant results between 2 techniques should prompt retesting in reference centers with in-house TBAs, particularly when the suspicion of an autoimmune or paraneoplastic syndrome is high.
Conflict of interest statement
C. Milano receives research support from Spanish National Health Institute Carlos III co-funded by the European Union (Rio-Hortega grant CM24/00055). C. Papi receives research support from Spanish National Health Institute Carlos III (FIS grant PI23/01366) and 2023 EAN Research Training Fellowship. L. Marmolejo receives research support from Spanish National Health Institute Carlos III (predoctoral research grant FI24/00021). M. Spatola receives research support from La Caixa Foundation (Junior Leader) and Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/01366). J. Dalmau receives research support from CaixaResearch Health 2022 (HR22-00221) and Spanish National Health Institute Carlos III (ISCIII) and co-funded by the European Union (FIS grant PI23/00858), Cellex Foundation, Fundació Clínic per a la Recerca Biomèdica (FCRB) Programa Multidisciplinar de Recerca, Generalitat de Catalunya Department of Health (SLT028/23/000071), Edmond J. Safra Foundation. He receives royalties from Euroimmun for the use of NMDA as an antibody test. He received a licensing fee from Euroimmun for the use of GABAB receptor, GABAA receptor, DPPX and IgLON5 as autoantibody tests; he has received a research grant from Sage Therapeutics. All the other authors report no disclosures relevant to the manuscript. Go to
Figures





Comment in
-
Beyond the Lab Slip: Why Laboratory Expertise Matters in Neuronal Antibody Testing and Why Clinicians Should Care.Neurol Neuroimmunol Neuroinflamm. 2025 Jul;12(4):e200425. doi: 10.1212/NXI.0000000000200425. Epub 2025 May 20. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 40393019 Free PMC article. No abstract available.
-
Assessing Commercial Tissue-Based Assays for Autoimmune Neurologic Disorders (II): Antibodies to Surface Antigens.Neurol Neuroimmunol Neuroinflamm. 2025 Jul;12(4):e200406. doi: 10.1212/NXI.0000000000200406. Epub 2025 May 20. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 40393020 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
