Hypertension, proteinuria, and RAAS inhibition use in children with systemic lupus erythematosus: Data from a multi-institutional pediatric learning health system
- PMID: 40393065
- PMCID: PMC12185231
- DOI: 10.1177/09612033251345201
Hypertension, proteinuria, and RAAS inhibition use in children with systemic lupus erythematosus: Data from a multi-institutional pediatric learning health system
Abstract
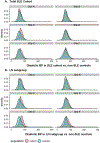
ObjectivesTo assess the potential of a multi-institutional pediatric learning health system for comparative effectiveness research in pediatric-onset systemic lupus erythematosus (SLE), we characterized renin angiotensin aldosterone system (RAAS) inhibitor utilization and the feasibility of ascertaining key treatment indications and outcomes, including hypertension and proteinuria.MethodsWe identified children with SLE and lupus nephritis (LN) at 6 PEDSnet institutions using previously developed computable phenotypes. A reference population of controls without SLE was randomly sampled from the same rheumatology/nephrology clinics (N = 200 controls per site). We evaluated data completeness, plausibility, and conformance related to RAAS inhibitor treatment indications and outcomes: (a) percent of encounters with blood pressure (BP) readings, (b) percent of BP readings with paired height, (c) percent of patients with ≥1 urinalysis within 7 days of SLE diagnosis, (d) urinalyses for which proteinuria could be classified as normal or abnormal, and (e) RAAS inhibitor use.ResultsThere were 1303 patients with SLE, including 457 with LN. BP measurements were available at 62% of encounters, of which 96% were paired with a height within 90 days. BP distributions were higher in patients with SLE and LN compared to controls without SLE. One third of SLE patients had a hypertension diagnosis. 60%-83% of patients at each site had a urinalysis protein measurement within 7 days of SLE diagnosis. A normal or abnormal result for proteinuria could be derived for 96% of measurements, and nearly all patients with LN had ≥1 abnormal result (range 94%-100% by site). RAAS inhibitors were prescribed to 31% of SLE and 71% of LN patients (range 60%-84% by site). Hypertension, elevated BP or proteinuria was identified in 90% of SLE cases at the time of RAAS inhibitor initiation.ConclusionWe demonstrated the feasibility of ascertaining health measurement data relevant to pediatric lupus treatment and outcomes in a pediatric learning health system, as well as hospital-level variation in RAAS inhibitor use. This work will facilitate more efficient multi-institutional comparative effectiveness research and also highlights opportunities for increased treatment standardization.
Keywords: Systemic lupus erythematosus; cardiovascular disease; renal lupus.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.C. serves on an advisory committee for the Childhood Arthritis and Rheumatology Research Alliance and is a member of a Data Safety Monitoring Board for an investigator-initiated clinical trial of an investigational treatment for SLE (NCT06839976) unrelated to this work. The remaining authors declare that there are no relevant conflicts of interest.
Figures


Similar articles
-
Short- and Long-Term Progression of Kidney Involvement in Systemic Lupus Erythematosus Patients with Low-Grade Proteinuria.Clin J Am Soc Nephrol. 2022 Aug;17(8):1150-1158. doi: 10.2215/CJN.01280122. Epub 2022 Jul 26. Clin J Am Soc Nephrol. 2022. PMID: 35882508 Free PMC article.
-
Validating claims-based algorithms for a systemic lupus erythematosus diagnosis in Medicare data for informed use of the Lupus Index: a tool for geospatial research.Lupus Sci Med. 2024 Oct 14;11(2):e001329. doi: 10.1136/lupus-2024-001329. Lupus Sci Med. 2024. PMID: 39401954 Free PMC article.
-
Renin-Angiotensin-Aldosterone System Modulators in Adults with Hypertension: A Network Meta-Analysis of Randomized Controlled Trials.Drugs. 2024 Nov;84(11):1445-1462. doi: 10.1007/s40265-024-02092-7. Epub 2024 Sep 23. Drugs. 2024. PMID: 39312177
-
Angiotensin-converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease.Cochrane Database Syst Rev. 2021 Dec 21;12(12):CD009191. doi: 10.1002/14651858.CD009191.pub4. Cochrane Database Syst Rev. 2021. PMID: 34932828 Free PMC article.
-
Enhancing systemic lupus erythematosus treatment outcomes with an early initiation of belimumab: insights from a multicenter retrospective study within the first five years.Arthritis Res Ther. 2025 May 29;27(1):116. doi: 10.1186/s13075-025-03581-0. Arthritis Res Ther. 2025. PMID: 40442811 Free PMC article.
References
-
- Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971–2013). Annals of the Rheumatic Diseases. 2019. Jun 1;78(6):802–6. - PubMed
-
- Khare R, Kappelman MD, Samson C, Pyrzanowski J, Darwar RA, Forrest CB, et al. Development and evaluation of an EHR-based computable phenotype for identification of pediatric Crohn’s disease patients in a National Pediatric Learning Health System. Learn Health Sys [Internet]. 2020. Oct [cited 2023 Aug 4];4(4). Available from: https://onlinelibrary.wiley.com/doi/10.1002/lrh2.10243 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

