Hypobaric hypoxia-driven energy metabolism disturbance facilitates vascular endothelial dysfunction
- PMID: 40393151
- PMCID: PMC12147897
- DOI: 10.1016/j.redox.2025.103675
Hypobaric hypoxia-driven energy metabolism disturbance facilitates vascular endothelial dysfunction
Abstract
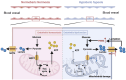
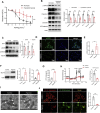
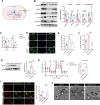
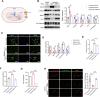
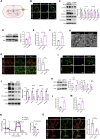
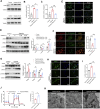
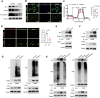
Hypobaric hypoxia in plateau environments inevitably disrupts metabolic homeostasis and contributes to high-altitude diseases. Vascular endothelial cells play a crucial role in maintaining vascular homeostasis. However, it remains unclear whether hypoxia-mediated changes in energy metabolism compromise vascular system stability and function. Through integrated transcriptomic and targeted metabolomic analyses, we identified that hypoxia induces vascular endothelial dysfunction via energy metabolism dysregulation. Specifically, hypoxia drives a metabolic shift toward glycolysis over oxidative phosphorylation in vascular endothelial cells, resulting in excessive lactate production. This lactate overload triggers PKM2 lactylation, which stabilizes PKM2 by inhibiting ubiquitination, forming a feedforward loop that exacerbates mitochondrial collapse and vascular endothelial dysfunction. Importantly, blocking the pyruvate-lactate axis helps maintain the balance between glycolysis and oxidative phosphorylation, thereby protecting vascular endothelial function under hypoxic conditions. Our findings not only elucidate a novel mechanism underlying hypoxia-induced vascular damage but also highlight the pyruvate-lactate axis as a potential therapeutic target for preventing vascular diseases in both altitude-related and pathological hypoxia.
Keywords: Energy metabolism; High-altitude; Hypobaric hypoxia; Lactate; PKM2; Vascular endothelial dysfunction.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. - PubMed
-
- Simon M.P., Tournaire R., Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J. Cell. Physiol. 2008;217:809–818. - PubMed
-
- Bosch-Marce M., Okuyama H., Wesley J.B., Sarkar K., Kimura H., Liu Y.V., et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007;101:1310–1318. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

