One-carbon homologation of alkenes
- PMID: 40393510
- PMCID: PMC12221989
- DOI: 10.1038/s41586-025-09159-9
One-carbon homologation of alkenes
Abstract
One-carbon homologues are structurally related and functionally identical organic molecules whose chain lengths differ by a single methylene (-CH2-) unit1. Across many classes of molecule-including pharmaceutical agents, natural products, agrochemicals, fragrances and petroleum products-the physicochemical characteristics exhibited by members of a homologous series subtly differ from one compound to another, which can impart remarkable differences to their function2. The efficient generation of homologues is, therefore, an important strategy in molecular discovery programmes3,4. Despite the availability of homologation strategies for several functional groups5,6, direct and general methods for one-carbon chain extension in alkenes remain an unmet synthetic need7,8. Here we report a catalytic one-carbon homologation process that is effective for many classes of alkene in simple and complex molecules. By leveraging the intrinsic reactivity of a new multifaceted allylsulfone reagent, a streamlined one-pot process, involving cross-metathesis and a fragmentation-retro-ene cascade, formally inserts a single methylene unit into the alkene chain. Among the applications of this process to several structurally and functionally complex molecules, we demonstrate how this practical transformation generates previously unexplored homologues of cyclosporine A9. These homologues exhibit modulated pharmacological and biological properties and could provide promising leads as cyclophilin inhibitors, a target that has great potential in many disease areas10.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



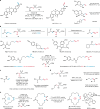
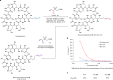

References
-
- Gerhardt, C. Recherches sur la Classification Chimique des Corps Organiques. Rev. Sci. Ind.14, 580–609 (1843).
-
- Lima, L. M., Alves, M. A. & do Amaral, D. N. Homologation: a versatile molecular modification strategy to drug discovery. Curr. Top. Med. Chem.19, 1734–1750 (2019). - PubMed
-
- Chen, D., Soh, C. K., Goh, W. H. & Wang, H. Design, synthesis, and preclinical evaluation of fused pyrimidine-based hydroxamates for the treatment of hepatocellular carcinoma. J. Med. Chem.61, 1552–1575 (2018). - PubMed
-
- Organ, H. M. et al. Novel derivatives at the C21 position of the FK-506 macrocycle. Bioorg. Med. Chem. Lett.3, 657–662 (1993).
-
- Sebastian, S., Monika, Khatana, A. K., Yadav, E. & Gupta, M. K. Recent approaches towards one-carbon homologation–functionalization of aldehydes. Org. Biomol. Chem.19, 3055–3074 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

