Impact of long-term non-invasive ventilation on severe exacerbations and survival in COPD: a French nationwide cohort study using multistate models
- PMID: 40393719
- PMCID: PMC12421116
- DOI: 10.1136/thorax-2024-222392
Impact of long-term non-invasive ventilation on severe exacerbations and survival in COPD: a French nationwide cohort study using multistate models
Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) is the most common indication for domiciliary non-invasive ventilation (NIV), but long-term outcomes data are limited.
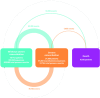
Objective: This multistate model analysis estimated the impact of NIV therapy continuation versus cessation on transitions between three different disease states.
Methods: Model data came from the French national health insurance reimbursement system database for individuals aged ≥40 years with COPD and ≥1 NIV reimbursement in 2015-2019.
Measurement and main results: Data from 49 503 patients started on NIV were included (median age 70 years, 51.2% male, median 1 exacerbation in the previous year). There were 80 361 severe exacerbations and 18 125 deaths (including 7805 in severe exacerbation). In multistate models, NIV continuation was associated with a significant reduction in transition to death, from severe exacerbation (HR 0.84, 95% CI 0.79 to 0.91) and without exacerbation (HR 0.88, 95% CI 0.83 to 0.93). NIV continuation versus cessation had no significant effect on transition between without exacerbation to severe exacerbation (HR 0.98, 95% CI 0.95 to 1.00) but was significantly associated with slower transition from severe exacerbation to without exacerbation (HR 0.87, 95% CI 0.84 to 0.89).
Conclusion: This multistate model analysis found that the long-term use of domiciliary NIV was associated with a lower risk of transitions to death, but was not associated with a reduction in recovery time after severe exacerbation. These data highlight the potential mortality benefits of long-term domiciliary NIV in COPD and can be used as one piece of evidence to support evidence-based guideline recommendations.
Keywords: COPD Exacerbations; COPD Pathology; Non invasive ventilation.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JLP has received lecture fees or conference travel grants from ResMed, Philips, AstraZeneca, Jazz Pharmaceuticals, Agiradom and Bioprojet, and has received unrestricted research funding from ResMed, Philips, GlaxoSmithKline, Bioprojet, Fondation de la Recherche Medicale (Foundation for Medical Research), Direction de la Recherche Clinique du CHU de Grenoble (Research Branch Clinic CHU de Grenoble), and fond de dotation 'Agir pour les Maladies Chroniques' (endowment fund 'Acting for Chronic Diseases'). AM is funded by the NIH. He reports income related to medical education from Livanova, Eli Lilly and Zoll, and ResMed provided a philanthropic donation to UCSD. PC has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding, has received research support from ResMed and SomnoMed and is a consultant to ResMed, SomnoMed, Signifier Medical Technologies, Bayer and Sunrise Medical. AB, FL and AJ are employees of ResMed. EH, HD and AS are employees of HEVA and their participation in this study was funded by ResMed. APa has received consulting fees from ResMed. APr has received investigator fees for clinical trials funded by ResMed. SB and JR have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
