Adenosine Kinase: An Epigenetic Modulator and Drug Target
- PMID: 40393929
- PMCID: PMC12092209
- DOI: 10.1002/jimd.70033
Adenosine Kinase: An Epigenetic Modulator and Drug Target
Abstract
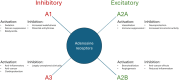
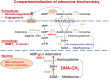
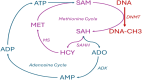
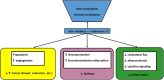
Adenosine kinase (ADK, EC: 2.7.1.20) is an evolutionarily ancient ribokinase, which acts as a metabolic regulator by transferring a phosphoryl group to adenosine to form AMP. The enzyme is of interest as a therapeutic target because its inhibition is one of the most effective means to raise the levels of adenosine and hence adenosine receptor activation. For these reasons, ADK has received significant attention in drug discovery efforts in the early 2000s for indications such as epilepsy, chronic pain, and inflammation; however, the report of adverse events regarding cardiovascular and hepatic function as well as instances of microhemorrhage in the brain of preclinical models prevented further development efforts. Recent findings emphasize the importance of compartmentalization of the adenosine system reflected by two distinct isoforms of the enzyme, ADK-S and ADK-L, expressed in the cytoplasm and the cell nucleus, respectively. Newly identified adenosine receptor independent functions of adenosine as a regulator of biochemical transmethylation reactions, which include DNA and histone methylation, identify ADK-L as a distinct therapeutic target for the regulation of the nuclear methylome. This newly recognized role of ADK-L as an epigenetic regulator points toward the potential disease-modifying properties of the next generation of ADK inhibitors. Continued efforts to develop therapeutic strategies to separate nuclear from extracellular functions of adenosine would enable the development of targeted therapeutics with reduced adverse event potential. This review will summarize recent advances in the discovery of novel ADK inhibitors and discuss their potential therapeutic use in conditions ranging from epilepsy to cancer.
Keywords: adenosine kinase; cancer; drug discovery; epigenetics; epilepsy; metabolism.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Detlev Boison is co‐founder and CDO of PrevEp Inc. Uchenna Peter‐Okaka declares that he has no conflicts of interest.
Figures
Similar articles
-
Adenosine kinase: An epigenetic modulator in development and disease.Neurochem Int. 2021 Jul;147:105054. doi: 10.1016/j.neuint.2021.105054. Epub 2021 May 5. Neurochem Int. 2021. PMID: 33961946 Free PMC article. Review.
-
Adenosine kinase: exploitation for therapeutic gain.Pharmacol Rev. 2013 Apr 16;65(3):906-43. doi: 10.1124/pr.112.006361. Print 2013 Jul. Pharmacol Rev. 2013. PMID: 23592612 Free PMC article. Review.
-
Adenosine Kinase: Cytoplasmic and Nuclear Isoforms.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 27. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 27. PMID: 39637220 Free Books & Documents. Review.
-
Adenosine kinase: A key regulator of purinergic physiology.Biochem Pharmacol. 2021 May;187:114321. doi: 10.1016/j.bcp.2020.114321. Epub 2020 Nov 6. Biochem Pharmacol. 2021. PMID: 33161022 Free PMC article. Review.
-
Adenosine kinase inhibition protects mice from abdominal aortic aneurysm via epigenetic modulation of VSMC inflammation.Cardiovasc Res. 2024 Sep 2;120(10):1202-1217. doi: 10.1093/cvr/cvae093. Cardiovasc Res. 2024. PMID: 38722818 Free PMC article.
References
-
- Oro J., “Mechanism of Synthesis of Adenine From Hydrogen Cyanide Under Possible Primitive Earth Conditions,” Nature 191 (1961): 1193–1194. - PubMed
-
- Oro J. and Kimball A. P., “Synthesis of Purines Under Possible Primitive Earth Conditions. I. Adenine From Hydrogen Cyanide,” Archives of Biochemistry and Biophysics 94 (1961): 217–227. - PubMed
-
- Newby A. C., “Adenosine and the Concept of Retaliatory Metabolites,” Trends in Biochemical Sciences 9 (1984): 42–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

