Cu(I)-Catalyzed Stereoselective Glycosylation of "Electron-Deficient" Glycals
- PMID: 40393946
- PMCID: PMC12131213
- DOI: 10.1021/acs.joc.5c00172
Cu(I)-Catalyzed Stereoselective Glycosylation of "Electron-Deficient" Glycals
Abstract
An efficient and mild Cu(I)-catalyzed Michael-type conjugate addition for 2-nitro glycals to access O-, S-, and C-glycosides with high stereoselectivity is reported. Under optimized conditions, nitrogalactals achieved high α-selectivity, whereas nitroglucal predominantly gave β-selective glycosides. The method is further demonstrated with other Michael-type substrates, including 2-formyl glycals and 3-keto glycals. Initial mechanistic investigations using NMR and supported by DFT calculations suggest that the reaction proceeds via a preorganized complex that positions the nucleophile close to the double bond to promote the Michael-type addition, in a manner analogous to enzyme-catalyzed processes. Moreover, the versatility of this synthetic approach was exemplified in the stereoselective synthesis of a mucin-type glycopeptide and the chemoselective one-pot synthesis of a trisaccharide.
Figures

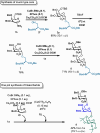


References
-
- Guberman M., Seeberger P. H.. Automated Glycan Assembly: A Perspective. J. Am. Chem. Soc. 2019;141(14):5581–5592. doi: 10.1021/jacs.9b00638. - DOI - PMC - PubMed
- Panza M., Pistorio S. G., Stine K. J., Demchenko A. V.. Automated Chemical Oligosaccharide Synthesis: Novel Approach to Traditional Challenges. Chem. Rev. 2018;118(17):8105–8150. doi: 10.1021/acs.chemrev.8b00051. - DOI - PMC - PubMed
- Wang L., Sorum A. W., Huang B. S., Kern M. K., Su G., Pawar N., Huang X., Liu J., Pohl N. L. B., Hsieh-Wilson L. C.. Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan-protein interactions. Nat. Chem. 2023;15(8):1108–1117. doi: 10.1038/s41557-023-01248-4. - DOI - PMC - PubMed
- Escopy S., Singh Y., Stine K. J., Demchenko A. V.. HPLC-Based Automated Synthesis of Glycans in Solution. Chem. - Eur. J. 2022;28(39):e202201180. doi: 10.1002/chem.202201180. - DOI - PMC - PubMed
- Roychoudhury, R. ; Pohl, N. L. B. . Light Fluorous-Tag-Assisted Synthesis of Oligosaccharides. In Modern Synthetic Methods in Carbohydrate Chemistry: From Monosaccharides to Complex Glycoconjugates; Werz, D. B. ; Vidal, S. , Eds.; Wiley: Weinhem, 2014; pp 221–239.
- Nielsen M. M., Pedersen C. M.. Catalytic Glycosylations in Oligosaccharide Synthesis. Chem. Rev. 2018;118(17):8285–8358. doi: 10.1021/acs.chemrev.8b00144. - DOI - PubMed
-
- Hainrichson M., Nudelman I., Baasov T.. Designer aminoglycosides: the race to develop improved antibiotics and compounds for the treatment of human genetic diseases. Org. Biomol. Chem. 2008;6(2):227–239. doi: 10.1039/B712690P. - DOI - PubMed
- Galan M. C., Dumy P., Renaudet O.. Multivalent glyco(cyclo)peptides. Chem. Soc. Rev. 2013;42(11):4599–4612. doi: 10.1039/C2CS35413F. - DOI - PubMed
LinkOut - more resources
Full Text Sources

