TREM2 deficiency exacerbates cognitive impairment by aggravating α-Synuclein-induced lysosomal dysfunction in Parkinson's disease
- PMID: 40393958
- PMCID: PMC12092616
- DOI: 10.1038/s41420-025-02538-1
TREM2 deficiency exacerbates cognitive impairment by aggravating α-Synuclein-induced lysosomal dysfunction in Parkinson's disease
Abstract
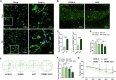
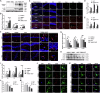
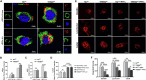
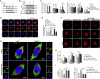
Cognitive impairment in Parkinson's disease (PD) is a widespread and rapidly progressive feature that impacts prognosis. Although TREM2 has been implicated in neuroprotection across various neurodegenerative diseases, its specific role in PD remains to be clarified. In this study, we first detected the hippocampus of human PD specimens and of the mutant A53T α-Synuclein transgenic mice (A53T mice), and found a significant increase in the number of TREM2+ microglia. To evaluate the effects of TREM2 deficiency, TREM2-deficient A53T mice (TREM2-/-/A53T mice) were generated. In these mice, exacerbated cognitive impairment, neurodegeneration, disruption of synaptic plasticity, and accumulation of pathological α-Synuclein (α-Syn) in the hippocampus were observed, without any detected motor dysfunction. Despite increased infiltration of activated microglia surrounding α-Syn aggregates, lysosomal dysfunction in microglia was aggravated in the TREM2-/-/A53T mice. In addition, transcriptional analyses and in vitro experiments further found that TREM2 knockdown inhibited the nuclear distribution of TFEB via the ERK1/2 pathway, exacerbating α-Syn-induced lysosomal dysfunction and causing more pathological α-Syn accumulation. Finally, HT22 cells were cocultured with TREM2 knockdown of BV-2 cells pretreated with recombinant human A53T α-Syn preformed fibrils (PFFs). The coculture experiments showed that TREM2 knockdown in BV-2 cells pretreated with PFFs enhanced the phosphorylation of α-Syn and promoted apoptosis in HT22 cells via inhibiting α-Syn degradation. In conclusion, TREM2 deficiency exacerbates cognitive impairment in PD by exacerbating α-Syn-induced microglial lysosomal dysfunction, identifying TREM2 as a potential therapeutic target.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. Paraffined human brain sections were obtained from the Forensic Identification Center of Southern Medical University (Guangzhou, China) with consent from the next of kin and approval from Ethics Committee of Forensic Identification Center of Southern Medical University. The animal experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Guangdong Provincial People’s Hospital.
Figures

Similar articles
-
Transmission of peripheral blood α-synuclein fibrils exacerbates synucleinopathy and neurodegeneration in Parkinson's disease by endothelial Lag3 endocytosis.Am J Physiol Cell Physiol. 2025 Mar 1;328(3):C836-C855. doi: 10.1152/ajpcell.00639.2024. Epub 2024 Dec 9. Am J Physiol Cell Physiol. 2025. PMID: 39652643
-
TREM2 deficiency aggravates α-synuclein-induced neurodegeneration and neuroinflammation in Parkinson's disease models.FASEB J. 2019 Nov;33(11):12164-12174. doi: 10.1096/fj.201900992R. Epub 2019 Aug 1. FASEB J. 2019. PMID: 31370707 Free PMC article.
-
TREM2 signaling in Parkinson's disease: Regulation of microglial function and α-synuclein pathology.Int Immunopharmacol. 2024 Dec 25;143(Pt 2):113446. doi: 10.1016/j.intimp.2024.113446. Epub 2024 Oct 29. Int Immunopharmacol. 2024. PMID: 39490141
-
Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson's disease.Glia. 2018 Aug;66(8):1752-1762. doi: 10.1002/glia.23338. Epub 2018 Apr 6. Glia. 2018. PMID: 29624735
-
p38-TFEB pathways promote microglia activation through inhibiting CMA-mediated NLRP3 degradation in Parkinson's disease.J Neuroinflammation. 2021 Dec 20;18(1):295. doi: 10.1186/s12974-021-02349-y. J Neuroinflammation. 2021. PMID: 34930303 Free PMC article.
References
-
- Tolosa E, Gaig C, Santamaría J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72:S12–20. - PubMed
-
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–13. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

