ACSS2 mediates an epigenetic pathway to regulate β-cell adaptation during gestation in mice
- PMID: 40393969
- PMCID: PMC12092658
- DOI: 10.1038/s41467-025-58322-3
ACSS2 mediates an epigenetic pathway to regulate β-cell adaptation during gestation in mice
Abstract
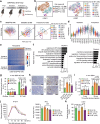
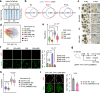
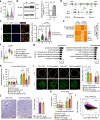
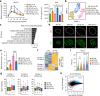
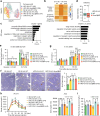
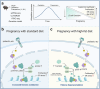
Maternal pancreatic β-cells undergo adaptive changes to meet the metabolic demands of pregnancy, and disruptions in this adaptation can lead to gestational diabetes mellitus. However, the mechanisms governing this adaptation remain largely unexplored. Using single-cell transcriptome combined with genetic analyses, we identified a precise process of β-cell adaptation in mice, characterized by progressive metabolic stress-related β-cell dysfunction, increased acetyl-CoA biosynthesis, and gene element-specific histone acetylation. STAT3 recruits p300 to promote histone acetylation of pregnancy-associated genes, a process enhanced by Acetyl-CoA Synthetase 2 (ACSS2). High-fat feeding induces hyperacetylation of chromatin regions specifically opened during pregnancy, leading to the overexpression of genes that impair β-cell function. However, these impairments can be rescued by β-cell-specific deletion of Acss2. Notably, ACSS2 is functionally implicated in the early establishment of β-cell adaptation in HFD-fed mice but does not appear to play a role in standard diet-fed mice until after the initiation of adaptation. Our study uncovers a finely regulated β-cell adaptation process at the single-cell level during pregnancy and identifies a specific epigenetic pathway that governs this process. These findings provide insights into β-cell plasticity and potential therapeutic strategies for gestational diabetes mellitus.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Banerjee, R. R. Piecing together the puzzle of pancreatic islet adaptation in pregnancy. Ann. N. Y Acad. Sci.1411, 120–139 (2018). - PubMed
-
- Brănişteanu, D. D. & Mathieu, C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol. Metab.14, 54–56 (2003). - PubMed
-
- Chiefari, E., Arcidiacono, B., Foti, D. & Brunetti, A. Gestational diabetes mellitus: an updated overview. J. Endocrinol. Investig.40, 899–909 (2017). - PubMed
-
- Park, S. & Kim, S. H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Diabetes62, A361–A361 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- 2024YFA1802900/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2023YFA1800600/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 32125014/National Natural Science Foundation of China (National Science Foundation of China)
- 32030034/National Natural Science Foundation of China (National Science Foundation of China)
- 32000566/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

