Epitope specificity of antibody-mediated protection induced in mice by the malaria vaccine RTS,S/AS01
- PMID: 40394009
- PMCID: PMC12092751
- DOI: 10.1038/s41541-025-01162-5
Epitope specificity of antibody-mediated protection induced in mice by the malaria vaccine RTS,S/AS01
Abstract
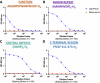
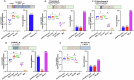
Antibodies induced by the malaria vaccine RTS,S/AS01 neutralize infectivity of transgenic sporozoites expressing Plasmodium falciparum CSP (PfCSP). These antibodies recognize the junctional, minor repeats, central repeats, and C-term regions of this antigen. The epitope specificity of antibodies mediating protection in mice was characterized in vivo using transgenic sporozoites expressing restricted antigenic portions of PfCSP. In this model, we found protection is mediated mostly by antibodies specific for the central repeats.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Author F.Z. serves as associate editor of this Journal and had no role in the peer review or decision to publish this manuscript. Author F.Z. declares no financial competing interest. All other authors declare no competing interests.
Figures
References
-
- W. H. O. W. m. r. World Health Organization. World malaria report 2022. https://www.who.int/teams/global-malaria-programme
-
- Gordon, D. M. et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis.171, 1576–1585 (1995). - PubMed
-
- Datoo, M. S. et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet403, 533–544 (2024). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

