Value of PET radiomic features for diagnosis and reccurence prediction of newly diagnosed oral squamous cell carcinoma
- PMID: 40394092
- PMCID: PMC12092716
- DOI: 10.1038/s41598-025-02305-3
Value of PET radiomic features for diagnosis and reccurence prediction of newly diagnosed oral squamous cell carcinoma
Abstract
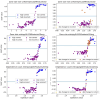
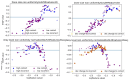
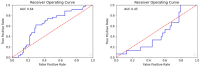
Oral Squamous Cell Carcinoma (OSCC) represents more than 90% of oral cancers. The usefulness of radiomic features extracted from PET images of OSCC patients to predict tumor characteristics such as primary tumor stage (T-stage), or tumor grade has not been investigated yet. In this prospective study, 112 patients with newly diagnosed, treatment-naïve OSCC were included. Tumor segmentation was performed using three strategies, the majority vote of these segmentations was used to calculate 445 radiomic features. Features instable over segmentation methods and features highly correlated with volume, SUVmax, and SUVmean were eliminated. A Random Forest classifier was trained to predict T-stage, tumor grade, lymph node involvement, and tumor recurrence. Stratified 10-fold cross-validation was performed. Evaluation metrics such as accuracy and area under the curve (AUC) were reported. SHAP dependence plots were generated to understand classifier decisions. The classifier reached a mean cross-validation AUC of 0.83 for predicting T-stage, an AUC of 0.56 for the grading of the primary tumor, a mean AUC of 0.64 for lymph node involvement, and a mean AUC of 0.63 for recurrence. In patients with newly-diagnosed OSCC, radiomics might have some potential to predict T-stage. These results need to be validated in a larger patient cohort.
Keywords: Positron emission tomography; Prediction; Radiomics; Squamous cell carcinoma of the oral cavity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The institutional review board of the Faculty of Medicine at the University of Würzburg approved this study, and written, informed consent was obtained from all participants (clinical trial number NCT04280159). The study was in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki.
Figures





References
-
- Disease Control Priorities, Third Edition (Volume 3): Cancer. 10.1596/978-1-4648-0349-9 (The World Bank, 2015). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

