The complexity of pharmaceutical expenditures across U.S. states
- PMID: 40394119
- PMCID: PMC12092636
- DOI: 10.1038/s41598-025-01885-4
The complexity of pharmaceutical expenditures across U.S. states
Abstract
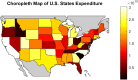
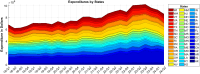
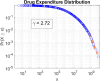
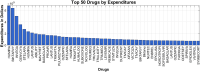
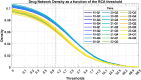
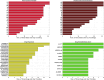
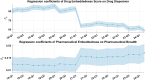
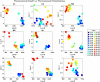
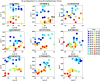
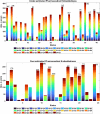
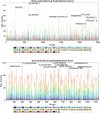
Understanding the complexity of pharmaceutical expenditures across U.S. states is critical for designing efficient healthcare policies and ensuring equitable drug access. This study applies network-based economic complexity methods to analyze state-level Medicaid drug spending, leveraging Medicaid State Drug Utilization Data (SDUD) from 2018 to 2024. Using Revealed Comparative Advantage (RCA) and the Method of Reflections, we quantify the sophistication of pharmaceutical consumption and identify structural inefficiencies in drug reimbursement policies. Our findings reveal substantial heterogeneity in pharmaceutical complexity across states, with highly diversified markets in states like California and Texas, while others, such as Wyoming and West Virginia, exhibit lower complexity due to restrictive formulary policies and healthcare infrastructure limitations. A 15% decline in network density over the study period suggests consolidation in reimbursement practices, influenced by regulatory interventions and cost-containment strategies. Additionally, Medicaid expansion states show a 20% increase in prescription utilization, particularly for antiviral and mental health medications. Null model comparisons highlight systematic deviations from expected expenditure patterns, with states like Arkansas and Nebraska showing lower-than-expected pharmaceutical embeddedness, whereas Massachusetts and California appear more integrated than network models predict. These findings suggest that state-specific policies, provider behavior, and market dynamics significantly shape pharmaceutical expenditures beyond structural network constraints, as well as they offer significant implications for policymakers and healthcare providers seeking to balance cost efficiency with equitable medication distribution.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Miller, R. D. & Frech, H. Is there a link between pharmaceutical consumption and improved health in oecd countries?. Pharmacoeconomics18, 33–45 (2000). - PubMed
-
- Sommers, B. D. & Oellerich, D. The poverty-reducing effect of medicaid. J. Health Econ.32, 816–832 (2013). - PubMed
-
- De Nardi, M., French, E. & Jones, J. B. Medicaid insurance in old age. Am. Econ. Rev.106, 3480–3520 (2016). - PubMed
-
- Sommers, B. D., Baicker, K. & Epstein, A. M. Mortality and access to care among adults after state medicaid expansions. N. Engl. J. Med.367, 1025–1034 (2012). - PubMed
-
- Sommers, B. D. & Grabowski, D. C. What is medicaid? more than meets the eye. JAMA318, 695–696 (2017). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

