Endometrial aging is accompanied by H3K27ac and PGR loss
- PMID: 40394215
- PMCID: PMC12092264
- DOI: 10.1038/s43587-025-00859-5
Endometrial aging is accompanied by H3K27ac and PGR loss
Abstract
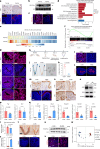
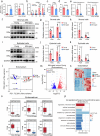
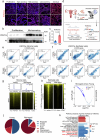
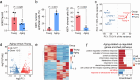
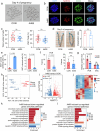
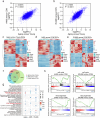
Whether and how endometrial aging affects fertility remains unclear. In our in-house clinical cohort at the Center for Reproductive Medicine of Peking University Third Hospital (n = 1,149), we observed adverse pregnancy outcomes in the middle-aged group after excluding aneuploid embryos, implying the negative impact of endometrial aging on fertility. To understand endometrial aging, we performed comprehensive transcriptomic profiling of the mid-secretory endometrium of young (<35 years) and middle-aged (≥35 years) patients. This analysis revealed that H3K27ac loss is linked to impaired endometrial receptivity in the middle-aged group. We eliminated H3K27ac in young human endometrial stromal cells and observed reduced progesterone receptor (PGR), a critical regulator of endometrial receptivity. Lastly, we validated the association between H3K27ac/PGR loss and uterine aging in a mouse model. Our findings establish H3K27ac as a critical regulator of PGR and demonstrate that endometrial H3K27ac loss is associated with aging-related fertility decline. This work provides valuable insights into enhancing the safety and efficacy of assisted reproductive technologies in future clinical practices.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Heffner, L. J. Advanced maternal age—how old is too old? N. Engl. J. Med.351, 1927–1929 (2004). - PubMed
-
- Sauer, M. V. Reproduction at an advanced maternal age and maternal health. Fertil. Steril.103, 1136–1143 (2015). - PubMed
-
- Frick, A. P. Advanced maternal age and adverse pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol.70, 92–100 (2021). - PubMed
-
- Beaujouan, É. & Toulemon, L. European countries with delayed childbearing are not those with lower fertility. Genus77, 2 (2021).
MeSH terms
Substances
Grants and funding
- 81925013/National Natural Science Foundation of China (National Science Foundation of China)
- 82288102/National Natural Science Foundation of China (National Science Foundation of China)
- 82301888/National Natural Science Foundation of China (National Science Foundation of China)
- 82225019/National Natural Science Foundation of China (National Science Foundation of China)
- 82192873/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

