Norcantharidin promotes M1 macrophage polarization and suppresses colorectal cancer growth
- PMID: 40394236
- PMCID: PMC12460879
- DOI: 10.1038/s41401-025-01578-8
Norcantharidin promotes M1 macrophage polarization and suppresses colorectal cancer growth
Abstract
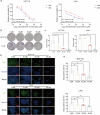
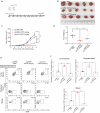
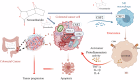
Colorectal cancer (CRC) is characterized by an immunosuppressive and inflammatory microenvironment, thus responds poorly to therapy. Previous studies show that norcantharidin (NCTD), a demethylated cantharidin (CTD) derived from Mylabris, exerts high efficacy in treating various cancers. In this study we investigated the antitumor effects of NCTD against CRC and the underlying mechanisms. Subcutaneous CRC models were established in balb/c mice using mouse colorectal cancer cell line CT26 and in balb/c nude mice using human colorectal cancer cell line HCT116. The mice were administered NCTD (2 or 4 mg·kg-1·d-1, i.p.) for 14 days. We showed that NCTD dose-dependently reduced the tumor growth in both the CRC models. Furthermore, NCTD markedly increased M1 macrophage infiltration in tumor tissue in both the CRC models. NCTD-induced macrophage M1 polarization was confirmed by flow cytometry and qPCR assays in both THP-1 cell-derived and RAW264.7 macrophage models in vitro. We demonstrated that NCTD (20, 40 μM) dose-dependently increased CSF2 secretion from CRC cells and macrophages, and suppressed the JAK2/STAT3 signaling pathway in CRC cells. Concurrently, NCTD (10-40 μM) dose-dependently inhibited CRC cell proliferation, invasion and migration in vitro. In conclusion, this study provides new evidence for the effects of NCTD against CRC and elucidates its antitumor mechanisms through remodeling the inflammatory microenvironment via CSF2-mediated macrophage M1 polarization and inhibiting JAK2/STAT3 phosphorylation in CRC cells.
Keywords: CSF2; JAK2/STAT3 pathway; M1 macrophage; colorectal cancer; norcantharidin; tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. - PubMed
-
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233–54. - PubMed
-
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–67. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

