Efficient removal of Remazol Red dye from aqueous solution using magnetic nickel ferrite nanoparticles synthesized via aqueous reflux
- PMID: 40394334
- PMCID: PMC12092790
- DOI: 10.1038/s41598-025-98478-y
Efficient removal of Remazol Red dye from aqueous solution using magnetic nickel ferrite nanoparticles synthesized via aqueous reflux
Abstract
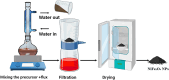
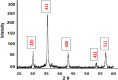
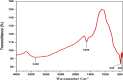
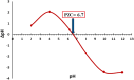
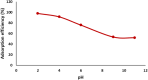
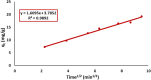
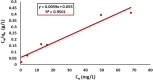
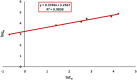
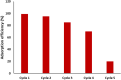
Rapid growth of the textile industry, along with the excessive use of water and dyes, has led to significant environmental concerns. This study introduces a straightforward, low-temperature aqueous reflux method for the fabrication of magnetic nickel ferrite (NiFe2O4) nanoparticles. The synthesized nanoparticles, characterized by X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and vibrating sample magnetometry (VSM), exhibited a cubic spinel structure, an average particle size of 23 ± 2.3 nm (range: 18-29.8 nm), and a magnetization of 56.96 ± 0.9 emu/g, enhancing their surface area and magnetic separability. These NiFe2O4 nanoparticles achieved a 96.5 ± 0.4% removal efficiency of Remazol Red dye from aqueous solutions after 90 min, with an adsorption capacity (qmax) of 169.5 ± 0.8 mg/g, as tested across pH 2-12, contact times of 10-120 min, and initial dye concentrations of 20-200 mg/L. Optimal removal occurred at pH 2, with a dye concentration of 20 mg/L and a 1 g/L dose, yielding 99 ± 0.5% efficiency, while adsorption decreased at high pH due to surface charge effects (PZC = 6.7). The results indicated that dye adsorption increased with decreasing pH and higher nickel ferrite dosage. Kinetic studies over 10-120 min followed pseudo-first-order (R2 = 0.96), Boyd, and Weber-Morris models, while isotherms across 20-200 mg/L conformed to the Freundlich model (R2 = 0.98), reflecting multilayer adsorption. These properties high crystallinity, nanoscale size, and strong magnetic responsiveness enhance the material's surface area, adsorption capacity, and ease of separation, contributing to its efficiency as an adsorbent. Reusability tests confirmed the stability of the nanoparticles and their consistent performance across multiple cycles. These results establish NiFe2O4 as an economical, magnetically separable, and ecologically sustainable adsorbent for wastewater treatment purposes.
Keywords: Nickel ferrite; Remazol red dye; Surface adsorption; Wastewater treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures













References
-
- Rahimi, S. M. et al. Application of CuFe2O4/CuS as a new green magnetic nanocomposite in adsorption of tetracycline from aqueous solutions: Mathematical models of thermodynamics, isotherms, and kinetics. Appl. Water Sci.15(1), 1–17 (2025).
-
- Hossein Panahi, A. et al. Photocatalytic degradation of humic acid using bentonite@ Fe3O4@ZnO magnetic nanocomposite: An investigation of the characterization of the photocatalyst, degradation pathway, and modeling by solver plugin. Water15(16), 2931 (2023).
-
- Fahim, Y.A. et al., Occupational Exposure to Heavy Metal Dust and Its Hazardous Effects on Non-ferrous Foundry Workers’ Health (2024).
-
- Mittal, R. et al. Surface modified novel synthesis of Spirulina assisted mesoporous TiO2@ CTAB nanocomposite employed for efficient removal of chromium (VI) in wastewater. Appl. Surf. Sci.679, 161309 (2025).
-
- Patar, S. et al. Algae derived N-doped mesoporous carbon nanoflakes fabricated with nickel ferrite for photocatalytic removal of Congo Red and Rhodamine B dyes. Surfaces Interfaces51, 104710 (2024).
LinkOut - more resources
Full Text Sources

