Rapamycin treatment for Alzheimer's disease and related dementias: a pilot phase 1 clinical trial
- PMID: 40394335
- PMCID: PMC12092812
- DOI: 10.1038/s43856-025-00904-9
Rapamycin treatment for Alzheimer's disease and related dementias: a pilot phase 1 clinical trial
Abstract
Background: Rapamycin has been shown to extend lifespan and acts on pathologies underlying Alzheimer's disease and related dementias in animal models. However, rapamycin's clinical application remains underexplored.
Methods: We conducted a single-site open-label phase 1 clinical trial (ClinicalTrials.gov: NCT04200911) to examine the effects of rapamycin in humans. Eligible participants were people 55-85 years old with mild cognitive impairment or early-stage dementia, which was defined as having a Global Clinical Dementia Rating Scale Score of 0.5-1. All participants received rapamycin (1 mg/day) for eight weeks. The primary aim was to evaluate rapamycin's central nervous system penetrance by assaying drug levels in the cerebrospinal fluid (CSF) before and after treatment. Secondary aims evaluated safety, cognition, Alzheimer's disease, and inflammatory biomarkers in the CSF and plasma.
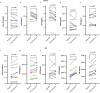
Results: In ten participants (mean age 74 ± 4 years, 60% female), we find that rapamycin is not detectable in the CSF before or after treatment. After treatment, we find that twenty, mostly mild adverse events occur, systolic blood pressure and hemoglobin A1c increase, multiple erythrocyte parameters decrease, and there are no significant cognitive changes. Furthermore, we find that CSF phosphorylated tau-181 (mean change (95% confidence interval) pg/ml), 2.64 [0.70-4.59]), glial fibrillary acidic protein (6262.21 [3787.44-9373.84]), and neurofilament light (367.19 [204.28-561.61]) and plasma interferon gamma (4.37 [3.01-5.74]), interleukin 5 (0.33 [0.12-0.64]), vascular endothelial growth factor D (3741.03 [1505.98-5976.07]), soluble fms-like tyrosine kinase-1 (258.88 [89.03-428.74]) and placental growth factor (20.81 [12.38-29.25]) significantly increase (FDR-corrected p-value < 0.05).
Conclusions: Rapamycin is not detectable in the CSF before or after treatment, but several Alzheimer's disease and inflammatory biomarkers increase after treatment. Our results highlight the need to better understand the biological effects and clinical impact of repurposing rapamycin for Alzheimer's disease.
Plain language summary
The drug rapamycin has been shown to increase longevity and reverse changes in the brain associated with Alzheimer’s disease and related dementias in animal models. However, rapamycin’s role in the clinical setting is unclear. Here we show data from a phase 1 clinical trial in ten participants with mild cognitive impairment or Alzheimer’s disease who were treated with rapamycin (1 mg/day) for eight weeks. Findings show that rapamycin levels were not detectable in cerebrospinal fluid before or after treatment. All participants knew they were receiving rapamycin and did not experience any serious negative health events due to the treatment. Additionally, several Alzheimer’s disease and inflammatory biomarkers were increased from baseline to post-treatment. These results highlight the need to better understand the impact of rapamycin on Alzheimer’s disease in humans.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Mitzi M. Gonzales reports personal stock in AbbVie. Miranda Orr has a patent, Biosignature and Therapeutic Approach for Neuronal Senescence, pending. Randall J. Bateman reports a relationship with C2N Diagnostics and receives income from serving on the scientific advisory board as a co-founder. Randall J. Bateman has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb, and Novartis. Washington University has equity ownership interest in C2N Diagnostics and receives royalty income based on technology (Stable Isotope Labeling Kinetics, Blood Plasma Assay, and Methods of Diagnosing AD with Phosphorylation Changes) licensed by Washington University to C2N Diagnostics. Dr. Seshadri has consulted for Eisai and Biogen outside the current work. All other authors declare no competing interests.
Figures

References
Associated data
Grants and funding
- U54AG07594/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- T32 AG021890/AG/NIA NIH HHS/United States
- U01 AG022307/AG/NIA NIH HHS/United States
- PG30AG044271/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- TR002647/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG077472/AG/NIA NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- R01 AG066524/AG/NIA NIH HHS/United States
- R01 AG033193/AG/NIA NIH HHS/United States
- R01NS095773/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- RF1AG061900/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 NS095773/NS/NINDS NIH HHS/United States
- R01 AG068293/AG/NIA NIH HHS/United States
- R01AG075684/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30AG013319/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 NS017950/NS/NINDS NIH HHS/United States
- R01AG054076/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01AG069690/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG054076/AG/NIA NIH HHS/United States
- RF1 AG059421/AG/NIA NIH HHS/United States
- R01HL105756/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01AG068293/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01 AG075684/AG/NIA NIH HHS/United States
- R01NS017950/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 AG069690/AG/NIA NIH HHS/United States
- R21 NS125171/NS/NINDS NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- RF1AG059421/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01AG077472/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R01AG066524/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U01AG22307/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- TL1 TR002647/TR/NCATS NIH HHS/United States
- P30AG066546/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21AG067559/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21 AG067559/AG/NIA NIH HHS/United States
- R01AG033193/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30 AG066546/AG/NIA NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
- I01 BX005717/BX/BLRD VA/United States
- RF1 AG061900/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

