Induction of more severe central sensitization in a medication overuse headache model mice through active ingestion of rizatriptan
- PMID: 40394497
- PMCID: PMC12090462
- DOI: 10.1186/s10194-025-02066-4
Induction of more severe central sensitization in a medication overuse headache model mice through active ingestion of rizatriptan
Abstract
Background: Medication overuse headache (MOH) is a secondary headache disorder arising from excessive use of acute analgesics in patients with primary headache. Current animal models that predominantly employ passive drug administration fail to recapitulate the hallmark feature of voluntary medication-seeking behaviour observed clinically. Therefore, we established a novel MOH mouse model with the active ingestion of rizatriptan (RIZ) to better simulate the clinical characteristics of MOH and explore changes in brain activation patterns.
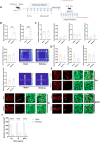
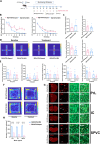
Methods: C57BL/6 J mice received intraperitoneal injections of nitroglycerin (NTG, 10 mg/kg) every other day. During the feeding period, they were provided with two bottles-one containing an RIZ solution (0.02 mg/kg) and the other containing deuterium depleted water (DDW)-allowing for voluntary intake. The bottle containing the RIZ solution was marked with a fixed colour indicator at the nozzle. Behavioural assessments included mechanical allodynia (von Frey filaments), anxiety-like behaviours (elevated plus maze, EPM and open field test, OFT), and drug-seeking quantification. Quantitative data from c-Fos immunostaining across 25 specific brain regions were subjected to Z score normalization, followed by three-tiered computational analyses: 1) hierarchical clustering (complete linkage) to characterize activation patterns, 2) Pearson correlation analysis for functional connectivity mapping, and 3) graph-theoretical network analysis (Cytoscape 3.2.1) to identify hub regions and their topological relationships. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonist, rimegepant (100 mg/kg, i.p., 7 injections) was administered during the modelling period, and withdrawal of RIZ and NTG was applied after modelling to observe behavioural and histological changes.
Results: Chronic RIZ consumption exacerbated NTG-induced cutaneous allodynia, prolonged central sensitization, and increased anxiety-like behaviour. Rimegepant attenuated allodynia progression, whereas withdrawal of RIZ and NTG normalized pain thresholds. Network analysis identified the prelimbic cortex (PrL) and spinal trigeminal nucleus caudalis (SPVC) as hub nodes. The PrL exhibited extensive functional connectivity with addiction-related regions (the insular cortex, IC and nucleus accumbens), whereas the SPVC showed predominant connections with pain-processing areas.
Conclusion: This study pioneers an ethologically valid MOH model that reflects more severe central sensitization and recapitulates active medication-seeking behaviour. PrL-mediated addiction-like-behaviour pathways and SPVC-centred nociceptive processing may play roles in the development of MOH. These findings provide novel neuromodulation targets (PrL, IC, SPVC) for refractory MOH management.
Keywords: Calcitonin gene-related peptide; Hierarchical clustering; Medication overuse headache; Network analysis; Rimegepant.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experimental procedures were approved by the Institutional Animal Care and Use Committee, Chinese PLA General Hospital, following the Regulations for the Administration of Affairs Concerning Experimental Animal. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Hagen K, Linde M, Steiner TJ, Stovner LJ, Zwart JA (2012) Risk factors for medication-overuse headache: an 11-year follow-up study. The nord-trøndelag health studies. Pain 153(1):56–61. 10.1016/j.pain.2011.08.018 - PubMed
-
- Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB (2008) Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 48(8):1157–1168. 10.1111/j.1526-4610.2008.01217.x - PubMed
-
- Radat F, Lanteri-Minet M (2010) What is the role of dependence-related behavior in medication-overuse headache? Headache 50(10):1597–1611. 10.1111/j.1526-4610.2010.01755.x - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

