Understanding the value of meningococcal vaccination for adolescents and young adults in the United States: insights from a steady-state modelling approach
- PMID: 40394570
- PMCID: PMC12090439
- DOI: 10.1186/s12889-025-21953-8
Understanding the value of meningococcal vaccination for adolescents and young adults in the United States: insights from a steady-state modelling approach
Abstract
Background: A two-dose series of quadrivalent meningococcal conjugate vaccine (MenACWY) is recommended for the prevention of invasive meningococcal disease (IMD) in adolescents in the United States. In June 2024, the Advisory Committee on Immunization Practices discussed plans to review the adolescent meningococcal vaccination schedule. Various options are under consideration, including removing the first dose of MenACWY at age 11-12 years.
Objectives: We evaluated the public health impact and cost-effectiveness of administering one or two doses of MenACWY compared to a scenario with no vaccination.
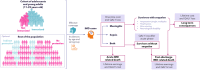
Methods: We constructed an incidence-based population model to compare costs and quality-adjusted life years (QALYs) associated with different vaccination schedules in a cohort of 11-25 year-olds, from a societal perspective, over a lifetime analytic horizon for outcomes related to death and disabilities. The main analyses compared various scenarios of MenACWY (Q) and MenB schedules to no vaccination. Further scenarios examined the impact of alternative assumptions applied to the first and/or second dose of MenACWY.
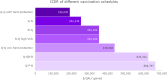
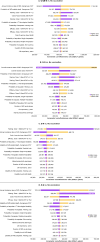
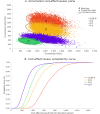
Results: Compared to no vaccination, 2 doses of MenACWY and 2 doses of MenB vaccine was projected to reduce IMD cases by 277 per year, resulting in an incremental cost-effectiveness ratio (ICER) of $625,322/QALY. Administering 2 doses of MenACWY was projected to reduce the annual number of IMD cases by 275 at an ICER of $438,948/QALY, which increased to 631 at an ICER of $190,030/QALY when herd immunity was considered. Alternatively, if only 1 dose of MenACWY was administered, the reduction in cases would be 253 if administered at 11-12 years old and 125 if given at 16 years, with ICERs of $252,249 per QALY and $352,169/QALY, respectively. Assuming a 25% increase in vaccination coverage rate, one MenACWY dose at 16 years resulted in 156 cases avoided.
Conclusions: The two doses of MenACWY that are currently recommended play a crucial role in reducing the burden of IMD and the first dose contributes significantly (≥ 90%) to this reduction. It is essential to take this finding into account when considering any updates to the adolescent meningococcal vaccination schedule in the United States.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: EL, CR, KG, AD, and TS are employees of Sanofi and may hold stock or stock options. JW currently holds a co-sponsored Industrial Research Chair grant with Sanofi. OC and EC are employees of Clever-Access, a consultancy that received funding from Sanofi to conduct this study.
Figures
References
-
- Meningococcal Disease. Signs and Symptoms [https://www.cdc.gov/meningococcal/about/symptoms.html]
-
- Meningococcal Disease. Causes and How It Spreads [https://www.cdc.gov/meningococcal/about/causes-transmission.html]
-
- Meningitis, Symptoms. [https://www.who.int/health-topics/meningitis#tab=tab_2]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

