Exploring the mechanism by which UCHL3 alleviates diabetic foot ulcers: FOXM1/NLRP3 inflammasome-mediated angiogenesis and endothelial cell pyroptosis
- PMID: 40394598
- PMCID: PMC12090482
- DOI: 10.1186/s13018-025-05914-w
Exploring the mechanism by which UCHL3 alleviates diabetic foot ulcers: FOXM1/NLRP3 inflammasome-mediated angiogenesis and endothelial cell pyroptosis
Abstract
Background: This study investigated the role of ubiquitin C-terminal hydrolase L3 (UCHL3) in regulating endothelial cell (EC) pyroptosis and angiogenesis in diabetic foot ulcers (DFUs), with a focus on FOXM1 and NLRP3 inflammasomes.
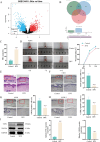
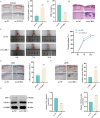
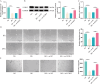
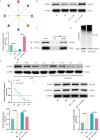
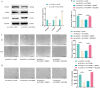
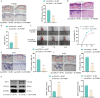
Methods: Differentially expressed genes in DFUs were identified using the GSE134431 dataset and cross-referenced with vascular formation-related factors from GeneCard and deubiquitinases from the UbiNet 2.0 database. A rat DFU model was used to evaluate wound healing, with or without UCHL3 overexpression and FOXM1 knockdown. Histological analysis and immunohistochemistry were employed to assess tissue morphology and the expression of CD31, eNOS, UCHL3, and FOXM1. In vitro, high glucose-induced human umbilical vein ECs (HUVECs) were transfected with UCHL3 overexpression and FOXM1 knockdown constructs. Cell viability, migration, and angiogenesis were assessed.
Results: UCHL3 expression was significantly reduced in DFU tissues. UCHL3 overexpression promoted wound healing in a rat model, while FOXM1 knockdown impaired wound healing and vascular formation. In HUVECs, UCHL3 overexpression enhanced cell viability, migration, and angiogenesis, accompanied by reduced NLRP3 and N-GSDMD levels. FOXM1 knockdown reversed these effects, but treatment with the NLRP3 inhibitor, MCC950, alleviated this damage.
Conclusion: UCHL3 enhances FOXM1 deubiquitination, inhibits NLRP3 inflammasome activation, and reduces EC pyroptosis, thereby contributing to DFU healing. UCHL3 and FOXM1 are potential therapeutic targets for DFU.
Keywords: Diabetic foot ulcers; Endothelial cells; FOXM1; NLRP3 inflammasome; UCHL3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This experiment has been approved by the Animal Ethics Committee of Hunan Evidence-based Biotechnology Co., Ltd. (ABTZ24002). All procedures and reporting were performed according to the ARRIVE guidelines including the 3R concept. Competing interests: The authors declare no competing interests.
Figures
References
-
- Ahluwalia R, Lazaro-Martinez JL, Reichert I, Maffulli N. Advances in pharmacotherapy for diabetic foot osteomyelitis. Expert Opin Pharmacother. 2021;22:2281–91. - PubMed
-
- Aicale R, Cipollaro L, Esposito S, Maffulli N. An evidence based narrative review on treatment of diabetic foot osteomyelitis. Surgeon. 2020;18:311–20. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

