From powerhouse to modulator: regulating immune system responses through intracellular mitochondrial transfer
- PMID: 40394666
- PMCID: PMC12090700
- DOI: 10.1186/s12964-025-02237-5
From powerhouse to modulator: regulating immune system responses through intracellular mitochondrial transfer
Abstract
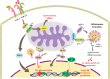
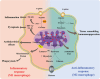
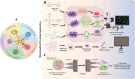
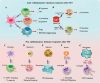
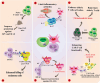
Mitochondria are traditionally known as the cells' powerhouses; however, their roles go far beyond energy suppliers. They are involved in intracellular signaling and thus play a crucial role in shaping cells' destiny and functionality, including immune cells. Mitochondria can be actively exchanged between immune and non-immune cells via mechanisms such as nanotubes and extracellular vesicles. The mitochondria transfer from immune cells to different cells is associated with physiological and pathological processes, including inflammatory disorders, cardiovascular diseases, diabetes, and cancer. On the other hand, mitochondrial transfer from mesenchymal stem cells, bone marrow-derived stem cells, and adipocytes to immune cells significantly affects their functions. Mitochondrial transfer can prevent exhaustion/senescence in immune cells through intracellular signaling pathways and metabolic reprogramming. Thus, it is emerging as a promising therapeutic strategy for immune system diseases, especially those involving inflammation and autoimmune components. Transferring healthy mitochondria into damaged or dysfunctional cells can restore mitochondrial function, which is crucial for cellular energy production, immune regulation, and inflammation control. Also, mitochondrial transfer may enhance the potential of current therapeutic immune cell-based therapies such as CAR-T cell therapy.
Keywords: Immune system; Immunometabolism; Immunotherapy; Mitochondria; Mitochondria Transfer; Organelle therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21(4):204–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

