Combining sodium-glucose co-transporter-2 inhibitor with mesenchymal stem cells and brown adipose tissue (BAT) and white adipose tissue (WAT) transplantation to mitigate the progression of diabetic kidney disease: a pre-clinical approach
- PMID: 40394690
- PMCID: PMC12093872
- DOI: 10.1186/s13287-025-04358-7
Combining sodium-glucose co-transporter-2 inhibitor with mesenchymal stem cells and brown adipose tissue (BAT) and white adipose tissue (WAT) transplantation to mitigate the progression of diabetic kidney disease: a pre-clinical approach
Abstract
Introduction: The increasing prevalence of Diabetes Mellitus (DM) correlates with a rising incidence of Diabetic Kidney Disease (DKD). DKD, a multifactorial condition, is characterized by activation of the renin-angiotensin-aldosterone system (RAAS), with angiotensin II playing a significant role in podocyte injury. While conventional treatments show potential in mitigating DKD progression, a combination of strategies is required to both impede its development and repair damaged structures.
Methods: In this study, we explored the brown adipose tissue (BAT) and white adipose tissue (WAT) transplantation, and the use of bone marrow mesenchymal stem cell therapy (BM-MSC) combined with sodium-glucose co-transporter-2 (SGLT2) inhibitor treatment and calorie restriction in the BTBRob/ob model, recognized as a robust representation of DKD featuring hyperglycemia, obesity, time-dependent albuminuria, and histological changes.
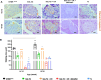
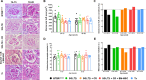
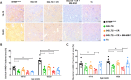
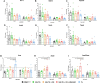
Results: Our primary findings revealed enhanced blood glucose control through combined cell therapy, diminished mesangial matrix expansion, alleviated tissue oxidative stress, preserved podocyte numbers, and an upregulation of podocyte structural markers and components of the RAAS renoprotective axis.
Conclusion: BM-MSC therapy demonstrates considerable promise as a combined treatment for mitigating DKD progression, with similar findings observed for BAT and WAT transplantation.
Keywords: Diabetic kidney disease; Mesenchymal stem cell; Podocyte; SGLT2i.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: Not applicable. Ethical approval and consent to participate: This research does not contain clinical experiments. All the experiments are approved under the projects “Treatment with empagliflozin, mesenchymal stem cells, and klotho as measures to slow down the progression of diabetic kidney disease and reduce the impact of acute kidney injury” and “Fat transplantation as a routine procedure to increase the number of BTBRob/ob mice in the animal facility” by the Institutional Animal Care and and Use Committee of Hospital Israelita Albert Einstein (HIAE). The approval numbers are SGPP 4900–21 (data of approval: August 27th, 2021) and SGPP 5171–22 (data of approval: April 29th, 2022), respectively. Our manuscript is reported following the ARRIVE guidelines.
Figures



References
-
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119. - PMC - PubMed
-
- Gnudi L. Renal disease in patients with type 2 diabetes: Magnitude of the problem, risk factors and preventive strategies. Presse Med. 2023;52: 104159. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

