Erk1/2 Orchestrates SSPH I-Induced Oxidative Stress, Mitochondrial Dysfunction and Ferroptosis in Hepatocellular Carcinoma
- PMID: 40394754
- PMCID: PMC12092237
- DOI: 10.1111/jcmm.70609
Erk1/2 Orchestrates SSPH I-Induced Oxidative Stress, Mitochondrial Dysfunction and Ferroptosis in Hepatocellular Carcinoma
Abstract
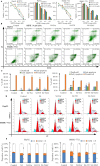
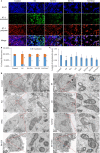
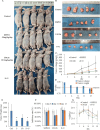
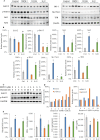
Although Erk1/2 has been linked to oxidative stress regulation in hepatocellular carcinoma (HCC), the interplay among Erk1/2, reactive oxygen species (ROS), and iron metabolism remains poorly characterised. The steroidal saponin SSPH I, a recognised ferroptosis inducer, exerts dual pharmacological effects via Erk1/2 and ROS-dependent pathways. This study aimed to investigate the regulatory mechanisms of Erk1/2 in ferroptosis and oxidative stress and analyse their feedback regulatory effects on Erk1/2 in HCC using SSPH I as a pharmacological probe, and further elucidate the anti-HCC effects and mechanisms of SSPH I in vitro and in vivo. Mechanistic studies utilised three inhibitors: U0126 (Erk1/2 phosphorylation inhibitor), Ferrostatin-1 (ferroptosis inhibitor), and N-acetyl cysteine (ROS scavenger), combined with SSPH I to delineate its effects on cell viability, mitochondrial dynamics, ferroptosis induction and oxidative stress. Mechanistically, SSPH I disrupted mitochondrial function and suppressed HCC cell survival through iron accumulation and ROS generation, while concurrently activating Erk1/2 signalling. Pharmacological inhibition of ROS or iron pathways partially attenuated SSPH I-induced ferroptosis and ROS generation, but failed to abrogate these effects. Erk1/2 inhibition completely abolished SSPH I-mediated regulation of the Nrf1/2-HO-1 axis and ferroptosis-related protein expression in cellular and animal models, identifying Erk1/2 as the upstream regulatory node. Notably, while both SSPH I and U0126 monotherapies inhibited xenograft growth, their combined use resulted in antagonistic effects. These findings establish Erk1/2 activation as the central molecular mechanism orchestrating SSPH I-driven oxidative stress amplification, mitochondrial dysfunction and ferroptosis execution in HCC.
Keywords: Erk1/2; Nrf1/2; ROS; SSPH I; ferroptosis.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

