A Systematic Review of Prolonged SARS-CoV-2 Shedding in Immunocompromised Persons
- PMID: 40394759
- PMCID: PMC12092234
- DOI: 10.1111/irv.70121
A Systematic Review of Prolonged SARS-CoV-2 Shedding in Immunocompromised Persons
Erratum in
-
Correction to "A Systematic Review of Prolonged SARS-CoV-2 Shedding in Immunocompromised Persons".Influenza Other Respir Viruses. 2025 Jun;19(6):e70127. doi: 10.1111/irv.70127. Influenza Other Respir Viruses. 2025. PMID: 40509676 Free PMC article. No abstract available.
Abstract
Background: Although reports have documented prolonged SARS-CoV-2 RNA detection in immunocompromised patients, few studies have systematically analyzed data on duration of SARS-CoV-2 in respiratory specimens of immunocompromised patients.
Methods: A systematic review was undertaken to describe SARS-CoV-2 RNA and infectious virus detection in immunocompromised patients from published data between January 1, 2020 and July 1, 2022. Patients were included if there was ≥ 1 positive SARS-CoV-2 RNA result in respiratory specimens collected > 20 days since symptom onset or first positive SARS-CoV-2 RT-PCR result.
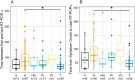
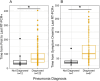
Results: Of the 183 patients, 175 were symptomatic with 83 (47.4%) that experienced intermittent relapsing symptoms, while pneumonia was reported in 122 (66.7%). Immunocompromising conditions represented were hematologic malignancy treatment (89, 48.6%), solid organ transplant (47, 25.7%), autoimmune disease treatment (14, 7.7%), solid tumor treatment (3, 1.6%), HIV infection (15, 8.2%), and primary immunodeficiency (15, 8.2%). Median duration from the first to the last positive SARS-CoV-2 RT-PCR result was 56 days in upper respiratory and 60 days in lower respiratory tract specimens. Significant differences in median duration of SARS-CoV-2 RNA detection were observed between patients with and without pneumonia and for patients with hematologic malignancies compared to solid organ transplant patients. Among patients with viral culture performed, median duration of replication-competent SARS-CoV-2 was 60.5 days from symptom onset (maximum 238 days) and 59 days from first RT-PCR positive result (maximum 268 days).
Conclusions: Immunocompromised persons can have replication-competent SARS-CoV-2 in respiratory tissues for months, including while asymptomatic. Serial SARS-CoV-2 testing can inform the duration of isolation for immunocompromised patients with SARS-CoV-2 infection.
Keywords: COVID‐19; SARS‐CoV‐2; immunocompromised.
Published 2025. This article is a U.S. Government work and is in the public domain in the USA. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
Shana Godfred‐Cato reports funding from HRSA for the Pediatric Pandemic Network to the University of Utah during completion of this study. All other authors declare no conflict of interest.
Figures

Similar articles
-
Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient.J Infect Chemother. 2021 Feb;27(2):387-389. doi: 10.1016/j.jiac.2020.12.001. Epub 2020 Dec 4. J Infect Chemother. 2021. PMID: 33328135 Free PMC article.
-
Duration of Replication-Competent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Shedding Among Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19).Clin Infect Dis. 2023 Feb 8;76(3):e416-e425. doi: 10.1093/cid/ciac405. Clin Infect Dis. 2023. PMID: 35607802 Free PMC article.
-
Prolonged SARS-CoV-2 Positivity in Immunocompetent Patients: Virus Isolation, Genomic Integrity, and Transmission Risk.Microbiol Spectr. 2021 Dec 22;9(3):e0085521. doi: 10.1128/Spectrum.00855-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787498 Free PMC article.
-
Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature.Infect Control Hosp Epidemiol. 2021 Jun;42(6):659-668. doi: 10.1017/ice.2020.1273. Epub 2020 Oct 20. Infect Control Hosp Epidemiol. 2021. PMID: 33077007 Free PMC article. Review.
-
SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis.Lancet Microbe. 2021 Jan;2(1):e13-e22. doi: 10.1016/S2666-5247(20)30172-5. Epub 2020 Nov 19. Lancet Microbe. 2021. PMID: 33521734 Free PMC article.
Cited by
-
Correction to "A Systematic Review of Prolonged SARS-CoV-2 Shedding in Immunocompromised Persons".Influenza Other Respir Viruses. 2025 Jun;19(6):e70127. doi: 10.1111/irv.70127. Influenza Other Respir Viruses. 2025. PMID: 40509676 Free PMC article. No abstract available.
References
-
- Cevik M., Tate M., Lloyd O., Maraolo A. E., Schafers J., and Ho A., “SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV Viral Load Dynamics, Duration of Viral Shedding, and Infectiousness: A Systematic Review and Meta‐Analysis,” Lancet Microbe 2, no. 1 (2021): e13–e22, 10.1016/s2666-5247(20)30172-5. - DOI - PMC - PubMed
-
- Wu Y., Guo Z., Yuan J., et al., “Duration of Viable Virus Shedding and Polymerase Chain Reaction Positivity of the SARS‐CoV‐2 Omicron Variant in the Upper Respiratory Tract: A Systematic Review and Meta‐Analysis,” International Journal of Infectious Diseases 129 (2023): 228–235, 10.1016/j.ijid.2023.02.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

