The Taproot Acts as a Storage Organ During Rapeseed Vernalization
- PMID: 40394819
- PMCID: PMC12092965
- DOI: 10.1111/ppl.70287
The Taproot Acts as a Storage Organ During Rapeseed Vernalization
Abstract
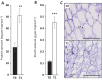
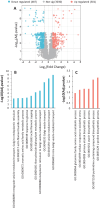
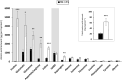
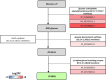
In winter oilseed rape (Brassica napus L.), vernalization, prolonged cold exposure, is essential for spring flowering. Although transcriptomic changes in leaves during vernalization are studied, the taproot, a key storage organ, remains unexplored. Recently, high nitrogen (N) and carbon (C) compound levels were observed in the taproot post-vernalization, suggesting potential metabolic activities in this organ during this period. To decipher this, an integrative study combining morphological, ionomic, proteomic, and targeted biochemical analysis was conducted. This study revealed that the taproot is the only compartment that shows net gain in biomass during vernalization and confirmed its role in storing C and N reserves. A comparative proteomic analysis between the beginning and the end of the vernalization period showed that this storage is the result of a strong modulation of proteins involved in N and C metabolisms. Additionally, the up-accumulation of proteins involved in the starch and amino acid metabolisms is consistent with the increase in the starch and amino acid amounts in the taproot during vernalization. Amino acids from the glutamine family are especially accumulated, with proline being the most over-accumulated (127-fold), highlighting the initiation of a protective metabolism in the taproot during the cold stress period related to vernalization. This study also reveals the storage of macro- and microelements, notably iron, copper, and zinc. These findings provide a deeper understanding of the development and maintenance of specific metabolic activities in the taproot of B. napus during vernalization, ensuring the accumulation of essential N and C reserves for subsequent growth and development.
Keywords: ionome; oilseed rape; proline; root proteome; starch.
© 2025 The Author(s). Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Multi-scale phenotyping of senescence-related changes in roots of rapeseed in response to nitrate limitation.J Exp Bot. 2025 Jan 10;76(2):312-330. doi: 10.1093/jxb/erae417. J Exp Bot. 2025. PMID: 39382543 Free PMC article.
-
Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: identification, characterization and immunolocalization of a putative taproot storage glycoprotein.J Exp Bot. 2002 Feb;53(367):265-75. doi: 10.1093/jexbot/53.367.265. J Exp Bot. 2002. PMID: 11807130
-
Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: effects of methyl jasmonate on nitrate uptake, senescence, growth, and VSP accumulation.J Exp Bot. 2002 May;53(371):1131-41. doi: 10.1093/jexbot/53.371.1131. J Exp Bot. 2002. PMID: 11971924
-
[Epigenetics of plant vernalization regulated by non-coding RNAs].Yi Chuan. 2012 Jul;34(7):829-34. doi: 10.3724/sp.j.1005.2012.00829. Yi Chuan. 2012. PMID: 22805208 Review. Chinese.
-
Molecular and biochemical mechanisms in maize endosperm development: the role of pyruvate-Pi-dikinase and Opaque-2 in the control of C/N ratio.C R Biol. 2008 Oct;331(10):772-9. doi: 10.1016/j.crvi.2008.07.019. Epub 2008 Sep 4. C R Biol. 2008. PMID: 18926491 Review.
References
-
- Avice, J.‐C. , and Etienne P.. 2014. “Leaf Senescence and Nitrogen Remobilization Efficiency in Oilseed Rape ( Brassica napus L.).” Journal of Experimental Botany 65: 3813–3824. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

