Islet Transplantation: Microencapsulation, Nanoencapsulation, and Hypoimmune Engineering
- PMID: 40394888
- PMCID: PMC12093044
- DOI: 10.1002/wnan.70016
Islet Transplantation: Microencapsulation, Nanoencapsulation, and Hypoimmune Engineering
Abstract
Islet transplantation represents a promising curative approach for type 1 diabetes by restoring glucose-responsive insulin secretion. However, the requirement for lifelong immunosuppression to prevent immune rejection can lead to significant side effects. Emerging strategies such as microencapsulation, nanoencapsulation, and hypoimmune engineering are being developed to protect transplanted islets from immune attack, thereby enhancing their viability and function. This review critically examines these innovative technologies, highlighting the methodologies, materials, experimental and clinical outcomes, as well as the challenges they face and potential solutions to overcome those challenges.
Keywords: cell encapsulation; hypoimmune engineering; islet transplantation; type 1 diabetes.
© 2025 The Author(s). WIREs Nanomedicine and Nanobiotechnology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


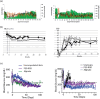




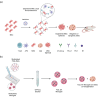
Similar articles
-
Use of additives, scaffolds and extracellular matrix components for improvement of human pancreatic islet outcomes in vitro: A systematic review.Islets. 2017 Sep 3;9(5):73-86. doi: 10.1080/19382014.2017.1335842. Epub 2017 Jul 5. Islets. 2017. PMID: 28678625 Free PMC article.
-
Challenges in Beta Cell Replacement for Type 1 Diabetes.Horm Res Paediatr. 2025;98(4):435-449. doi: 10.1159/000542206. Epub 2024 Oct 30. Horm Res Paediatr. 2025. PMID: 39476804 Review.
-
Innovations in bio-engineering and cell-based approaches to address immunological challenges in islet transplantation.Front Immunol. 2024 Apr 8;15:1375177. doi: 10.3389/fimmu.2024.1375177. eCollection 2024. Front Immunol. 2024. PMID: 38650946 Free PMC article. Review.
-
Immune-evasive beta cells in type 1 diabetes: innovations in genetic engineering, biomaterials, and computational modeling.Front Immunol. 2025 Aug 19;16:1618086. doi: 10.3389/fimmu.2025.1618086. eCollection 2025. Front Immunol. 2025. PMID: 40904460 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
-
- Alagpulinsa, D. A. , Cao J. J. L., Driscoll R. K., et al. 2019. “Alginate‐Microencapsulation of Human Stem Cell‐Derived β Cells With CXCL12 Prolongs Their Survival and Function in Immunocompetent Mice Without Systemic Immunosuppression.” American Journal of Transplantation 19, no. 7: 1930–1940. - PubMed
-
- Alipal, J. , Mohd Pu'ad N. A. S., Lee T. C., et al. 2021. “A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation.” Materials Today Proceedings 42: 240–250.
-
- Arakaki, R. , Yamada A., Kudo Y., Hayashi Y., and Ishimaru N.. 2014. “Mechanism of Activation‐Induced Cell Death of T Cells and Regulation of FasL Expression.” Critical Reviews in Immunology 34, no. 4: 301–314. - PubMed
-
- Bhaiji, T. , Zhi Z. L., and Pickup J. C.. 2012. “Improving Cellular Function and Immune Protection via Layer‐By‐Layer Nanocoating of Pancreatic Islet β‐Cell Spheroids Cocultured With Mesenchymal Stem Cells.” Journal of Biomedical Materials Research ‐ Part A 100A, no. 6: 1628–1636. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

